Hyperosmotic and hypertonic solutions are two terms that are often used interchangeably in biology. However, these terms have distinct meanings that are important to understand. In this article, we will explore the differences between hyperosmotic and hypertonic solutions and why hyperosmotic solutions are always hypertonic.
Hyperosmotic solutions are solutions that have a higher concentration of solutes than another solution. This means that the total amount of solutes in the hyperosmotic solution is greater than that in the other solution. The osmotic pressure of a hyperosmotic solution is higher than that of the other solution. Osmotic pressure is a measure of the force that drives water molecules to move from an area of low solute concentration to an area of high solute concentration. In other words, water molecules will move from a hypotonic solution (low solute concentration) to a hyperosmotic solution (high solute concentration) in order to balance the concentration of solutes on either side of the membrane.
Hypertonic solutions, on the other hand, are solutions that have a higher concentration of solutes than another solution and case cells to shrink. A hypertonic solution has a higher osmotic pressure than a hypotonic solution (low solute concentration) or an isotonic solution (equal solute concentration). When a cell is placed in a hypertonic solution, water molecules move out of the cell in order to balance the concentration of solutes on either side of the membrane. This causes the cell to shrink and can eventually lead to cell death.
It is important to note that hyperosmotic solutions are always hypertonic. This is because a hyperosmotic solution has a higher concentration of solutes than another solution, which means that it will always have a higher osmotic pressure than the other solution. If a hyperosmotic solution is placed in a hypotonic solution, water molecules will move into the hyperosmotic solution in order to balance the concentration of solutes on either side of the membrane. This means that the hypotonic solution will become hypertonic and the hyperosmotic solution will remain hyperosmotic.
Hyperosmotic and hypertonic solutions are two terms that are often used interchangeably in biology. However, it is important to understand the differences between these terms. Hyperosmotic solutions have a higher concentration of solutes than another solution and a higher osmotic pressure. Hypertonic solutions are solutions that cause cells to shrink and have a higher osmotic pressure than a hypotonic or isotonic solution. Hyperosmotic solutions are always hypertonic because they have a higher osmotic pressure than another solution.
Are Hypertonic and Hyperosmotic the Same?
Hypertonic and hyperosmotic are related terms, but they are not exactly the same. A hypertonic solution is one that has a higher concentration of solutes than another solution, while hyperosmotic describes a solution that has a higher osmotic pressure than another solution.
In simpler terms, hypertonicity refers to the effect on the cells, while hyperosmolality refers to the effect on the fluid surrounding the cells.
For example, if a cell is placed in a hypertonic solution, water will move out of the cell to try to balance the concentration of solutes between the inside and outside of the cell. This can cause the cell to shrink or even die. On the oher hand, if a cell is placed in a hyperosmotic solution, water will move out of the cell, but the cell may not necessarily shrink if the solutes cannot pass through the cell membrane.
Hypertonicity and hyperosmolality are related but different concepts that describe the effects of solutions on cells and their surrounding fluids.
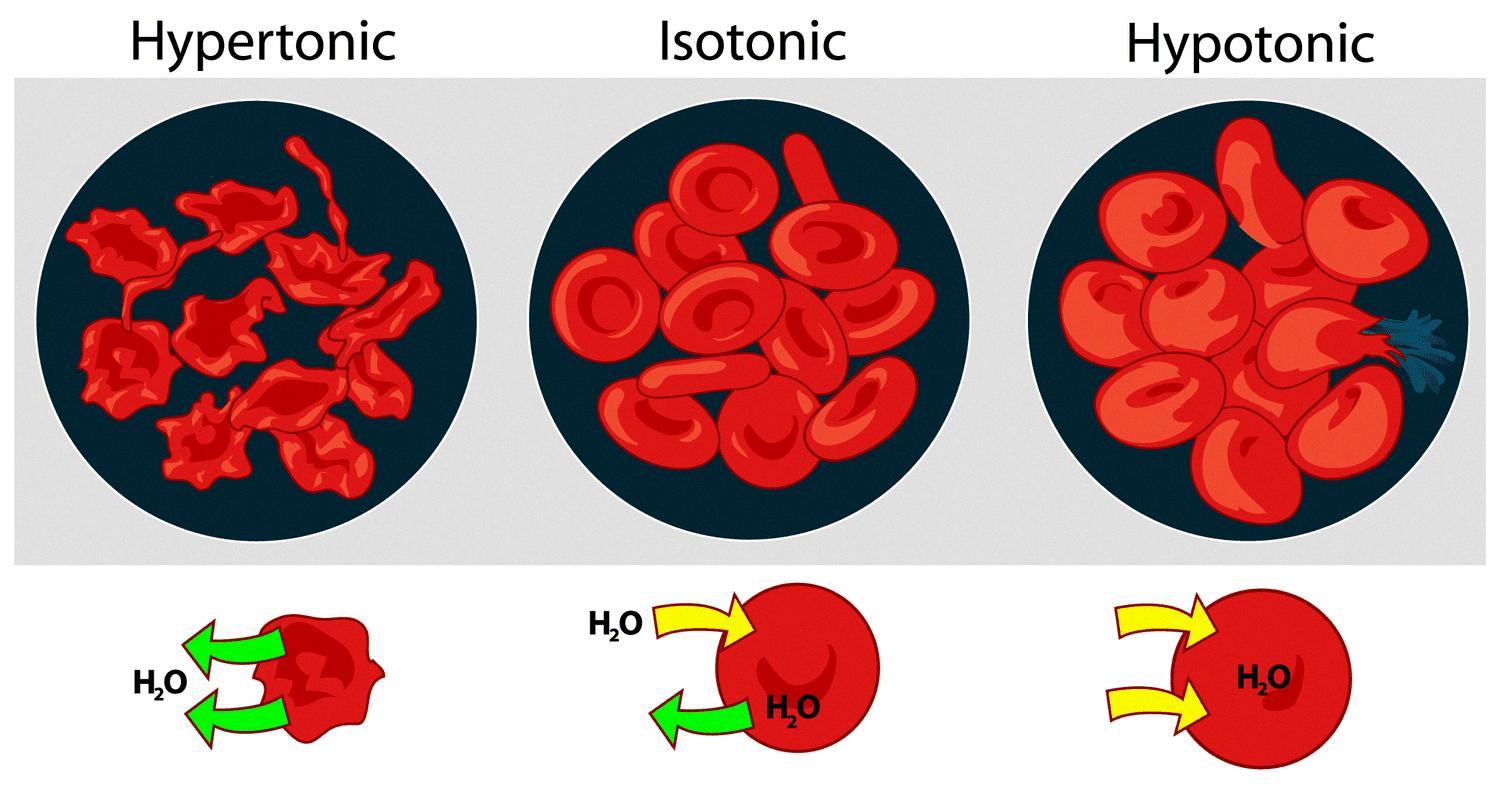
Source: thoughtco.com
Understanding Hypertonic and Hyperosmotic Solutions
Hypertonic, also referred to as hyperosmotic, is a term used in biology to describe a solution with an increased osmotic pressure, which is typically higher than the physiological level. Osmotic pressure refers to the pressure that a solution exerts when it is separated from another solution by a semipermeable membrane, which allows only cerain molecules to pass through.
In a hypertonic solution, the total amount of solutes, which are substances dissolved in the solution, including both permeable and impermeable solutes, is greater than that of another solution. This difference in solute concentration creates an osmotic pressure gradient, causing water molecules to move from a region of lower solute concentration to a region of higher solute concentration through the semipermeable membrane.
The movement of water molecules from an area of low solute concentration to high solute concentration results in a net movement of water out of the cell, leading to cell shrinkage or even cell death in extreme cases. This process is known as plasmolysis.
Hypertonic solutions are commonly used in laboratory experiments to induce plasmolysis in plant cells or to lyse (burst) bacterial cells. In contrast, hypotonic solutions have a lower solute concentration than another solution, while isotonic solutions have an equal solute concentration as another solution.
Hypertonic, or hyperosmotic, refers to a solution with an increased osmotic pressure, which can have significant effects on cells and biological processes.
The Effects of Hyperosmotic Solutions
Hyperosmotic solution is a term used to describe a solution that has a higher concentration of solutes compared to another solution. In other words, it is a solution that has a greater osmotic pressure than the surrounding solution.
For instance, if we compare saltwater and freshwater, saltwater wold be considered hyperosmotic. This is because saltwater has a higher concentration of solutes (salt) compared to freshwater. When a cell from a saltwater fish is placed in freshwater, the cell is said to be hyperosmotic to the freshwater.
Hyperosmotic solutions can have different effects on cells, depending on the type of cell and the concentration of the solution. In some cases, hyperosmotic solutions can cause cells to shrink or even die due to the loss of water through osmosis. In other cases, cells may adapt to the hyperosmotic environment by changing their shape or pumping solutes out of the cell to maintain osmotic balance.
It is important to note that hyperosmotic solutions are not limited to saltwater and freshwater. They can also be found in various biological systems, such as the human body. For example, if the concentration of salt in the blood becomes too high, it can lead to hyperosmotic dehydration, which can have serious health consequences.
To summarize, a hyperosmotic solution is one that has a higher concentration of solutes compared to another solution. This can have various effects on cells and biological systems, and it is important to understand the concept of osmotic pressure to fully appreciate the role of hyperosmotic solutions in biology.
Hypertonicity of a Hyperosmotic Solution
A hyperosmotic solution refers to a solution that has a higher concentration of solutes compared to another solution. Hypertonicity, on the othr hand, is a measure of the osmotic pressure gradient between two solutions separated by a semipermeable membrane. Thus, a hyperosmotic solution may or may not be hypertonic, depending on the relative concentrations of solutes in the two solutions.
To determine if a hyperosmotic solution is hypertonic, we need to compare the solute concentrations of the solution and the cell. If the solute concentration of the solution is higher than that of the cell, and the solutes cannot cross the membrane, the solution will be hypertonic to the cell. This will result in a net movement of water out of the cell, causing it to shrink or even die in extreme cases.
It is important to note that the terms hyperosmotic and hypertonic are not interchangeable. A solution can be hyperosmotic without being hypertonic, and vice versa. For example, a solution containing only non-penetrating solutes can be hyperosmotic to a cell, but if the solutes can freely cross the membrane, the solution will not be hypertonic to the cell.
A hyperosmotic solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane.
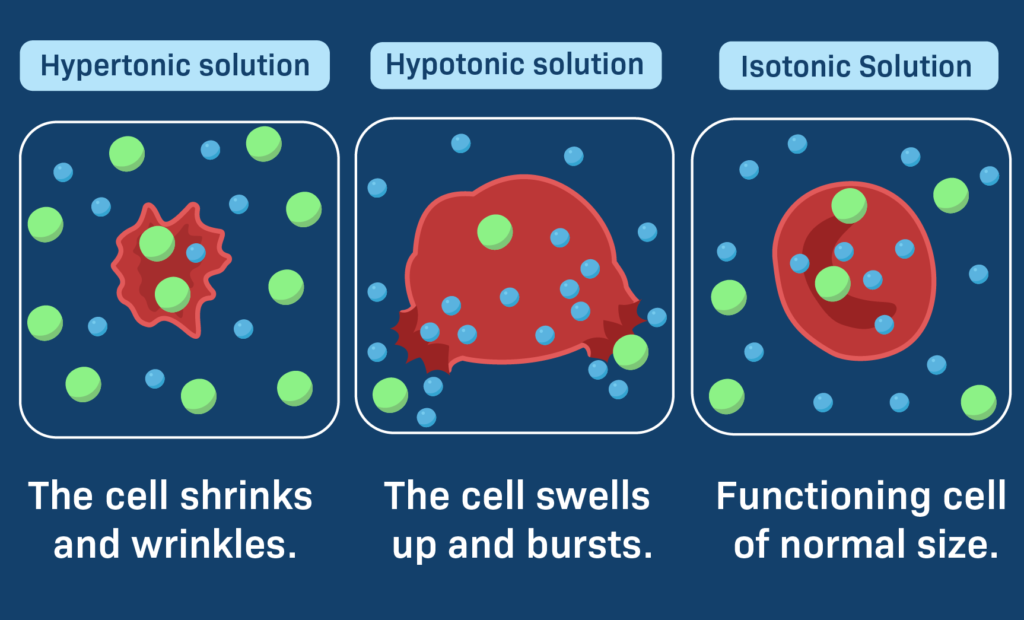
Conclusion
Hyperosmotic stress is a condition where the extracellular environment has a higher concentration of solutes than the intracellular environment. This can lead to cell shrinkage or swelling, which can have detrimental effects on cellular function. It is important to understand the causes and mechanisms of hyperosmotic stress, as it can occur in a variety of biological settings, including during dehydration or in diseases such as diabetes. By understanding the underlying mechanisms, researchers can develop strategies to mitigate the effects of hyperosmotic stress and improve overall cellular health.
