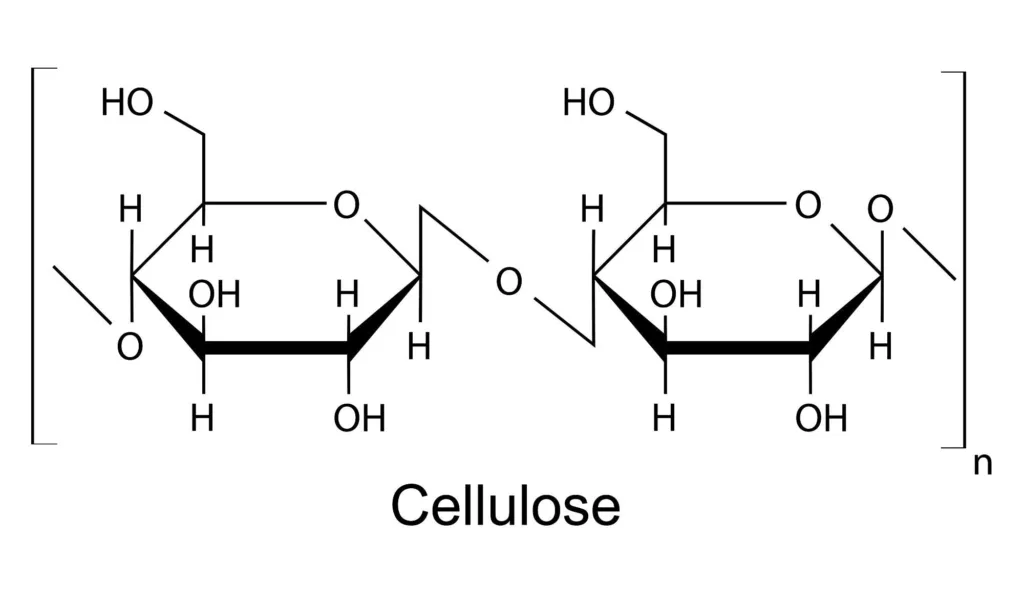
Cellulose is a complex organic molecule made up of beta-glucose monomers, where the O-H group on carbon one points up. The beta-glucose monomers in cellulose produce a nearly-linear molecule that is used as a structural component in plants. It is the most abundant organic molecule on earth, since it is the main component of plant cell walls. Wood, paper, and cotton are the most common forms of cellulose.
The glucose units in cellulose are linked together by beta glycosidic bonds, which are diffeent than the alpha glycosidic bonds found in glycogen and starch. This means that the O-H group on carbon one of one glucose molecule is linked to the O-4 group on carbon four of the next glucose molecule, resulting in a long, straight chain conformation.
Cellulose is a highly important molecule in the world of plant biology, as it provides the structural support needed for plants to stand upright and grow tall. Without cellulose, plants would not be able to withstand the forces of wind and rain, and would be unable to compete with other plants for resources.
In addition to its role in plant structure, cellulose has a variety of other uses in industry and manufacturing. It is commonly used in the production of paper, textiles, and even food products. Because of its strong, fibrous nature, it is also a popular material for building and construction.
So, to answer the question at hand: cellulose has beta glycosidic linkage, which makes it different from glycogen and starch, both of which have alpha glycosidic bonds. This key difference in the way the glucose units are linked together is what gives cellulose its unique properties and makes it such an important molecule in the natural world.
Is Cellulose a Type of Alpha or Beta Glucose?
Cellulose is made up of beta-glucose monomers. In contrast, alpha-glucose monomers make up other important carbohydrates such as starch and glycogen. The distinction between alpha- and beta-glucose lies in the orientation of the hydroxyl (-OH) group attached to the first carbon atom. In alpha-glucose, the -OH group points downwards, while in beta-glucose, it points upwards. In cellulose, the beta-glucose monomers form a linear chain, where each monomer is linked to the next through a beta-1,4 glycosidic bond. These bonds create a strong and rigid structure that makes cellulose an essential component of plant cell walls.
Does Cellulose Contain Alpha Bonds?
Cellulose does not have alpha bonds. Instead, it is composed of glucose units linked together by beta glycosidic bonds. This structural difference is significant, as it affects the way in which enzymes can break down and digest cellulose. In contrast, glycogen and starch both contain alpha bonds, which make them more easily digestible by the human body. Cellulose is an important structural component of plant cell walls, and is the most abundant organic molecule on earth. It is found in a wide range of materials, including wood, paper, and cotton.
Does Cellulose Have a Beta 1/4 Linkage?
Cellulose does have a Beta 1/4 linkage. In fact, it is the specific type of linkage that makes up the cellulose polymer. These linkages occur between the glucose monomers in the cellulose chain, resulting in a linear, unbranched structure. This Beta 1/4 linkage also makes cellulose very resistant to digestion by most animals, as they lack the necessary enzymes to break down this type of linkage. Instead, cellulose is used as a structural component in plants, providing rigidity and strength to cell walls.
Does Cellulose Contain Alpha Glucose?
Cellulose does not contain alpha glucose. Instead, it is composed of beta glucose monomers that are linked together by beta-1,4 glycosidic bonds. This structural difference is what makes cellulose indigestible by most animals, as the enzymes in their digestive systems cannot break down these beta linkages. In contrast, starch, which is another polysaccharide found in plants, is composed of alpha glucose monomers linked together by alpha-1,4 glycosidic bonds. This difference in structure also leads to different functions for these two polysaccharides in plants, with starch serving as a storage form of glucose and cellulose prviding structural support in cell walls.
Does Cellulose Contain Alpha Glucose?
Cellulose molecules do not have alpha glucose. Cellulose is a polysaccharide that is composed of beta-glucose units linked together by beta-1,4-glycosidic bonds. In contrast, starch, whih is another polysaccharide, is composed of alpha-glucose units linked together by alpha-1,4-glycosidic bonds. The difference in the orientation of the hydroxyl group on carbon 1 of the glucose monomers gives rise to distinct physical and chemical properties of these two polysaccharides. Cellulose is a fibrous and rigid material that is insoluble in water, while starch is a more amorphous and water-soluble substance that serves as a storage form of glucose in plants. the presence or absence of alpha glucose in a polysaccharide molecule is an important determinant of its structure, function, and biological roles.
Source: nature.com
Types of Bonds Present in Cellulose
Cellulose is a complex carbohydrate and a linear polymer composed of repeating units of d-glucopyranose linked by β-1,4-glycosidic bonds. These bonds are formed by the condensation reaction between the hydroxyl group (-OH) of the C4 carbon atom of one glucose molecule and the hydroxyl group (-OH) of the C1 carbon atom of the adjacent glucose molecule. The β-1,4-glycosidic bonds in cellulose are very stable and difficult to break, which gives cellulose its high tensile strength and resistance to degradation.
Bonding of Cellulose
The bonds that join cellulose molecules are covalent C-O-C linkages, which are flanked by two hydrogen bonds. This bond geometry implies a certain degree of cooperativity between covalent and hydrogen bonding. The covalent C-O-C linkage forms a strong, stable bond that provides structural integrity to the cellulose chain, while the hydrogen bonds between adjacent chains contribute to the overall strength and stability of the cellulose structure. It is worth noting that the unique arrangement of these bonds in cellulose gives it unique properties, such as its high tensile strength and insolubility in water.
The Bonds That Compose Cellulose
Cellulose is a complex linear polymer glucan that is composed of glucose units. The glucose units in cellulose are linked by β-(1–4)-glycosidic bonds, which are strong covalent bonds that connect the individual glucose molecules together in a linear chain. These bonds are unique to cellulose and prvide the structural rigidity and strength that are characteristic of this biopolymer. In addition to their important structural role, the β-(1–4)-glycosidic bonds in cellulose also make the molecule resistant to degradation by many enzymes, which helps to ensure its long-term stability and durability. the β-(1–4)-glycosidic bonds are a critical component of cellulose and play an essential role in its unique properties and functions.
Types of Glycosidic Bonds
Glycosidic bonds can exist as either alpha or beta glycosidic bonds. The type of glycosidic bond formed depends on the stereochemistry of the two carbons involved in the bond. An alpha-glycosidic bond is formed when both carbons have the same stereochemistry, meaning that the hydroxyl group on the anomeric carbon is positioned below the plane of the ring, while in a beta-glycosidic bond, the two carbons have diferent stereochemistry, meaning that the hydroxyl group on the anomeric carbon is positioned above the plane of the ring. The type of glycosidic bond is important because it affects the conformation of the resulting molecule, which in turn can affect its function and properties.
Types of Carbohydrates With β Glycosidic Linkage
Carbohydrates that have β glycosidic linkage include lactose, cellobiose, and maltose. Lactose is a disaccharide consisting of one molecule of β-galactose and one molecule of α-glucose linked togther by a β-1,4-glycosidic bond. Cellobiose is a disaccharide composed of two β-glucose molecules linked by a β-1,4-glycosidic bond. Maltose is a disaccharide comprising two α-glucose molecules linked by a β-1,4-glycosidic bond. The β glycosidic linkage refers to the orientation of the anomeric carbon of the monosaccharide residue involved in the bond formation. In β glycosidic linkage, the anomeric carbon of one sugar residue is linked to the hydroxyl group on the fourth carbon of the other sugar residue in a trans configuration.
β 4 Glycosidic Linkages: Where Are They Found?
β-1,4-glycosidic linkages are commonly found in complex carbohydrates such as starch, glycogen, and cellulose. In starch, β-1,4-glycosidic linkages are the primary bonds that connect glucose monomers in linear chains, while branching in the polysaccharide is created by α-1,6-glycosidic linkages. The β-1,4-glycosidic linkages in glycogen are similar to thoe in starch, but with more frequent branching. In contrast, in cellulose, β-1,4-glycosidic linkages connect glucose monomers in a linear fashion to form long chains, which are then cross-linked via hydrogen bonds to create a rigid structure. β-1,4-glycosidic linkages play a critical role in the structure and function of complex carbohydrates in nature.
Compound Containing a β 1 → 4 Linkage
The compound that contins a β 1 → 4 linkage is lactose. Lactose is a disaccharide composed of two monosaccharides, namely, galactose and glucose. The linkage between the two monosaccharides is formed by a β-1,4-glycosidic bond, which refers to the specific type of covalent bond that joins the two sugar molecules together. This type of linkage is important in the structure and function of carbohydrates, as it determines the shape, stability, and chemical properties of the molecule. The β 1 → 4 linkage in lactose is a key factor in the digestion and metabolism of this carbohydrate, as it is broken down by the enzyme lactase, which specifically cleaves this bond to release the individual monosaccharides for absorption in the body.
Glycosidic Linkage of β1 → 4 Carbohydrates
A β1 → 4 glycosidic linkage is a type of chemical bond between two monosaccharides, in which the anomeric carbon of one monosaccharide is linked to the fourth carbon of the other monosaccharide. This linkage is found in several carbohydrates, including lactose, which is a disaccharide made up of galactose and glucose. Therefore, lactose has a β1 → 4 glycosidic linkage between the galactose and glucose units. Other carbohydrates that have this type of linkage include cellobiose and maltose. These linkages play a crucial role in the structure and function of carbohydrates, affecting their physical properties and biological activities.
The Number of Glycosidic Bonds in Cellulose
Cellulose contains 1-4 glycosidic bonds between glucose molecules. The 1-4 glycosidic bond is a covalent bond that forms between the carbon-1 of one glucose molecule and the carbon-4 of the neighboring glucose molecule. This bond creates a linear chain of glucose molecules that are linked together by strong hydrogen bonds between adjacent chains. Cellulose is a complex polysaccharide that consists of thousands of glucose molecules linked together via these 1-4 glycosidic bonds. The linear arrangement of cellulose chains makes it a strong and durable molecule, which is why it is an important structural component in plant cell walls.
Conclusion
Cellulose is a complex polysaccharide made of beta-glucose monomers that form a nearly-linear molecule. It is the most abundant organic molecule on earth and is found in plant cell walls, wood, paper, and cotton. Cellulose is a structural component in plants and is made up of beta-1,4-glycosidic linkages that give it a long, straight chain conformation. The unique beta glycosidic bonds betwen glucose units make cellulose resistant to digestion in most animals, but it plays a critical role in the digestive systems of animals like cows and termites. Cellulose is a fascinating molecule with important applications in a variety of industries, including textiles, paper, and biofuels. Understanding the properties and structure of cellulose is essential for scientists and researchers in fields ranging from biochemistry to material science.
