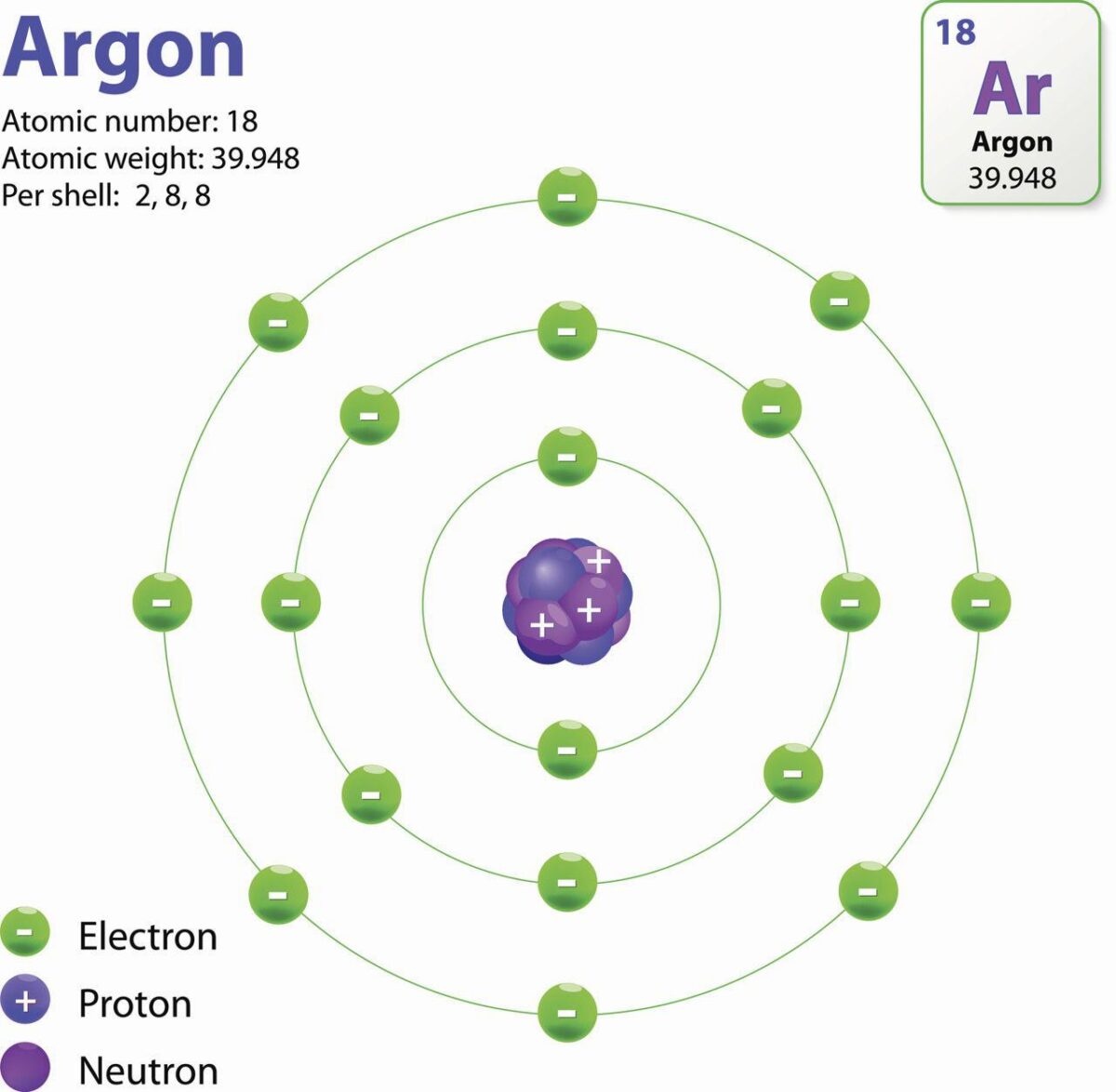
The elements that make up all of the matter in our universe are composed of three subatomic particles: protons, neutrons, and electrons. Argon is a noble gas that has two significant characteristics when it comes to these particles. First, it has a full octet of electrons in its outer shell, meaning it doesn’t react with other elements and thus doesn’t gain or lose any electrons. Second, the number of protons and neutrons in an element are equal; thus, argon also has 22 neutrons.
Understanding the properties of protons, neutrons, and electrons is essential for comprehending the composition and behavior of various elements on earth and beyond. This blog will provide an overview of what these particles are and how they interact with each other in argon specifically.
Protons are positively charged particles located at the center of atoms known as nuclei. They have a mass equivalent to one atomic mass unit (amu) and interact with other atoms through electrostatic forces to form chemical bonds. Neutrons are found in the nucleus along with protons but don’t carry any electrical charge; they’re neutral particles that account for almost half of an atom’s mass. Electrons are negatively charged particles orbiting around the nucleus; they account for most of an atom’s volume but very little mass.
In argon specifically there are 22 protons (equal to the number of neutrons) creating a stable atom due to its complete octet of electrons in its outer shell. As previously mentioned this makes argon unreactive with other elements as it cannot gain or lose any electrons – thus leaving it unchanged over time – while simultaneously making up part of many compounds due to its electron configuration being able to form strong intermolecular forces with other atoms.
As such, understanding how protons, neutrons and electrons interact in argon is key for comprehending how this element behaves as part of different compounds or mixtures on earth or elsewhere in space – something which could potentially be used for various applications ranging from medicine to engineering depending on the situation at hand.
What Is The Neutrons Of Argon?
The neutrons of argon are 22. This is beause argon is a noble gas and has a complete octet of electrons in its outer shell. This means that it doesn’t react with other elements, so it doesn’t lose or gain any electrons. The number of neutrons in an element is the same as the number of protons, so the number of neutrons in argon is also 22.
What Element Has 18 Protons And 22 Neutrons?
The element with 18 protons and 22 neutrons is argon. Argon is a noble gas, wich means it is inert. This means that it does not react with other elements.
How Many Protons And Neutrons Does Argon 40 Have?
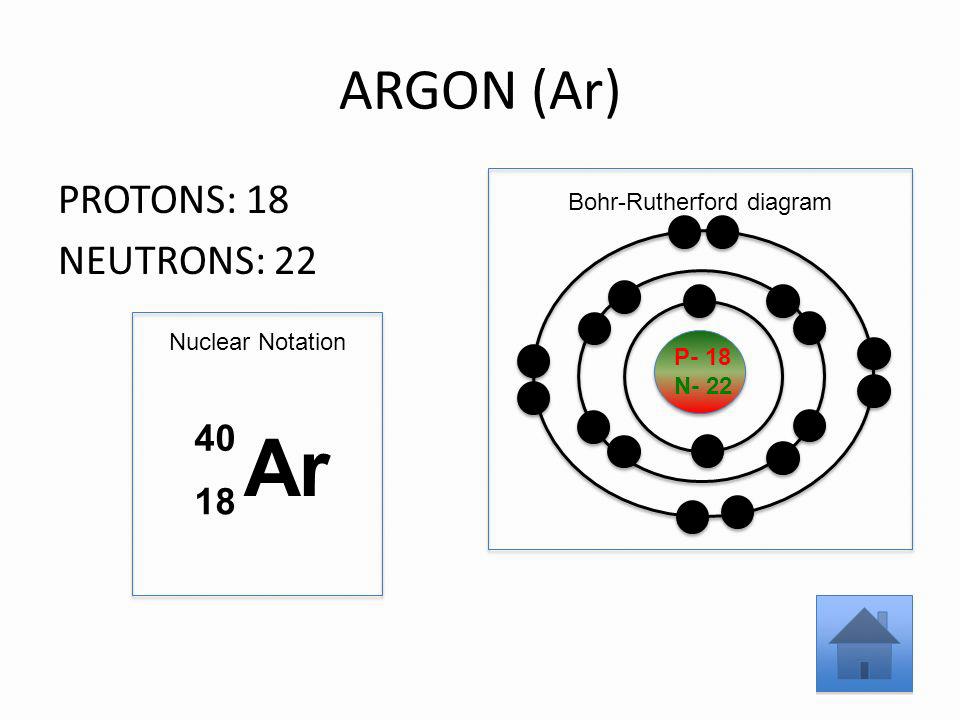
Argon-40 has 18 protons and 22 neutrons.
Why Number Of Neutrons In Argon Are 22?
The number of neutrons in argon is 22 because that is the atomic number of argon. The atomic number is the number of protons in an atom. Since, a neutral atom of argon also has 22 Neutrons. So, it is clear that this is not an isotope but a neutral atom of Argon.
What Isotope Of Argon Has 22 Neutrons?
The isotope of argon with 22 neutrons is 40Ar. This is because the number of neutrons in an atom of a particular isotope determines the atomic weight of that isotope.
Which Element Contains 84 Protons?
Polonium is an element that contains 84 protons. It was discovered in 1898 by Marie Curie and her husband Pierre Curie. Polonium is a very radioactive element and it is not found in nature in its pure form.
How Many Neutrons Does Argon 36?
Argon-36 has 18 neutrons.
How Many Neutrons Are In Argon 41?
The atomic number of argon is 18 and the mass number is 40. There are 22 neutrons in argon 41.
How Many Neutrons Does Argon 37 Have?
Argon-37 has 18 protons and 19 neutrons in its nucleus. This makes it very similar to argon-36, whch has a nucleus comprising 18 protons and 17 neutrons.
How Many 3s Electrons Are In Argon?
There are six 3s electrons in Argon.
Which Is The Correct Electron Configuration Of Argon 18?
The electron configuration of Argon 18 is 1s22s22p63s23p6.
How Many Protons And Neutrons Are In An Atom Of 38 Ar?
An atom of 38 Ar has 18 protons and 20 neutrons in it.
How Many Protons Neutrons And Electrons Are In An Argon 39 Atom?
Argon-39 is an atom with 18 protons, 22 neutrons and 18 electrons. It is a stable atom and does not undergo radioactive decay.
What Has 9 Protons And 9 Neutrons?
The element fluorine has 9 protons and 9 neutrons. In the image on the right it has gained a neutron and now has 10. Since it has 10 particles, but only 9 positive ones, its net charge is –1. A negatively charged ion is called an anion.
What Is Argon Gas Formula?
Argon Gas has an atomic number of 18 and a molecular weight of 39.948 g/mol. It is a noble gas that is monoatomic and is used in fluorescent tubes. The boiling point of argon gas is ?185.848 °C and the melting point is ?189.34 °C.
