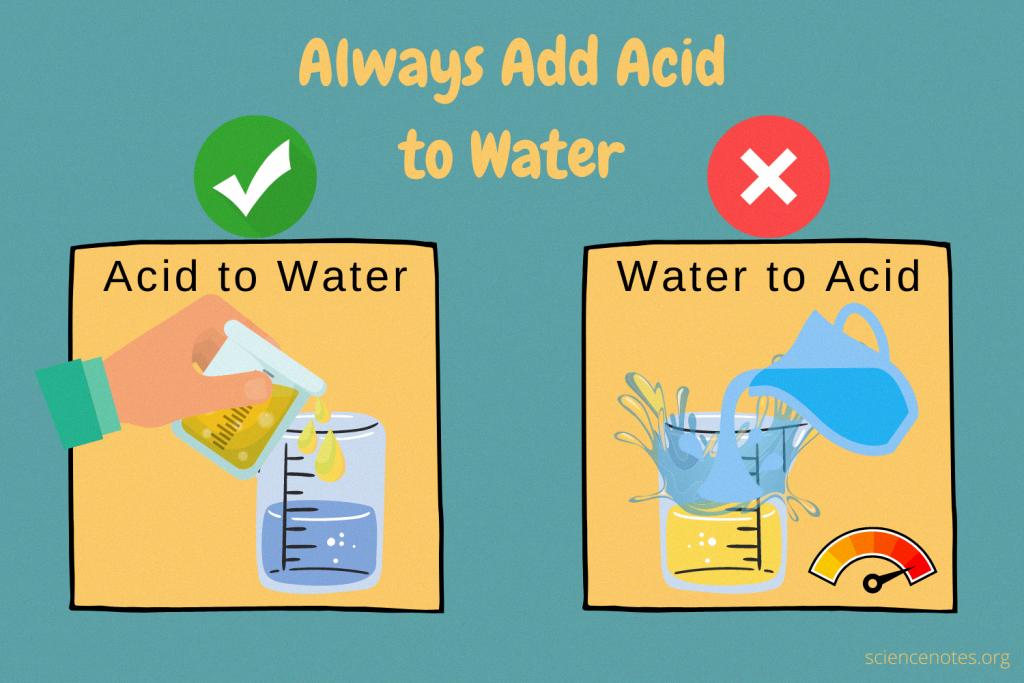
Acid is an important component of many industrial and household products, but it can be dangerous if handled improperly. One of the most important safety rules when working with acid is to remember that acid should always be added to water, not the othr way around.
When adding acid to water, the acid must be added slowly and with constant stirring, as the reaction between them is exothermic (producing heat). If too much heat is generated, it can cause the mixture to splash out and cause burns. Adding water to acid instead can also cause splattering or splashing of the acid which is dangerous.
It’s important to understand why adding acid to water is preferable. When an acid is dissolved in water, it produces hydrogen ions which increase the concentration of hydrogen ions in solution. This process produces heat and is known as enthalpy of solution or enthalpy of dissolution. The exothermic nature of this reaction means that if you add too much acid too quickly, there may be enough heat generated for the mixture to splash out which could be dangerous.
For these reasons, it’s essential that when working with acids you take proper safety precautions and always add acids to water rather than the oher way around. It’s also important that acids are added slowly and with constant stirring in order to reduce the amount of heat generated by the reaction. By taking these simple steps you can ensure your safety when using acids!
Adding Acid to Water but Not Water to Acid
Adding acid to water rther than water to acid is the safest way to mix the two substances. When water is added to a concentrated acid, the reaction is exothermic and can produce a large amount of heat, which can cause the mixture to splatter or even boil. This can be dangerous, as it increases the risk of burns from coming into contact with the hot liquid. Conversely, when an acid is added slowly to water with constant stirring, the reaction produces less heat and is more manageable. Therefore, adding an acid to water ensures that any potential hazards are minimized.
Source: thoughtco.com
The Effects of Adding Acid to Water
Yes, it is correct to add acid to water when mixing them together. This is because adding water to acid can cause the acid to splatter and splash up, which can be dangerous and damaging. To mix strong acids and water safely, always add the acid to the water slowly and carefully. This will help minimize risk of injury or damage from splattering or spills.
Which Should Come First: Acid or Water?
When mixing acid and water, it is important to always add the acid to the water. Acid is a much more reactive substance than water, so when combining them, the heat generated (known as enthalpy of solution or enthalpy of dissolution) can be dangerous if the acid is added to the water after it has been poured into a container. For safety, it is best practice to always add the acid first and then slowly pour in the water whle stirring for even distribution. This will help avoid any potential harm from heat released during mixing.
Reaction of Acid to Water
Acid reaction to water is a chemical process in which an acid, when dissolved in water, produces hydrogen ions, increasing the concentration of hydrogen ions in the solution. This reaction is highly exothermic, meaning that it releases heat energy, as the production of hydrogen ions requires energy. During the process, the acid molecule breaks down into its components, with one part becoming a positively charged hydrogen ion and anothr part becoming a negatively charged ion. The result is an increase in acidity or a decrease in pH. In some cases, this reaction may also produce carbon dioxide gas bubbles as a byproduct.
Adding Acid to Water or Water to Acid: Which is Better?
No – you should never add acid to water. When diluting acids, it is important to always pour the acid slowly into the water and stir to get rid of any generated heat. Pouring water into concentrated acid can caue a dangerous reaction, so it is important to remember that acid should always be added to water.
Source: sciencenotes.org
Order of Acids
The correct order of acid strength for the given compounds is alkyne > alkene > alkane. Alkynes are the most acidic hydrocarbons due to the presence of a triple bond, which is more polar than a double bond in an alkene or single bond in an alkane. This increased polarity leads to increased electron density around the carbon atom, making it more likely to donate electrons and form a hydrogen ion when reacting with water. Alkenes, on the other hand, have decreased acidity due to the double bond havng less polarity than the triple bond in an alkyne. Lastly, alkanes do not contain any double or triple bonds and thus have very low acidity since they cannot donate electrons towards forming hydrogen ions.
The Order of Acids
The order of acid strength, from strongest to weakest, is: perchloric acid, hydrochloric acid, hydrobromic acid, hydroiodic acid, sulphuric acid, nitric acid, and chloric acid. Perchloric acid is the strongest of the strong acids because it completely dissociates in water to form hydrogen ions (H+). Hydrochloric acid is next in terms of strength because it also completely dissociates in water to form H+. Hydrobromic and hydroiodic acids are both weaker than hydrochloric but still classified as strong acids. Sulphuric and nitric acids are even weaker than the previous three and are often referred to as medium-strong acids. Chloric acid is the weakest of all the strong acids snce it only partially dissociates in water.
Order of Acidity
The actual order of acidity for carbon atoms is spCH?CH > CH3?spC?CH > sp2CH2=CH2 > sp3CH3?CH3. This is because the s-character of the carbon atom increases in the order sp > sp2 > sp3. As the s-character of the carbon atom increases, its acidic strength also increases.
A carbocation (a positively charged carbon atom) is more stable when it has more s-character, and a higher degree of s-character increases its acidity. Therefore, aong all the given molecules/ions, the one with highest s-character (i.e., spCH?CH) has highest acidity and that with lowest s-character (i.e., sp3CH3?CH3) has lowest acidity.
Reaction of an Element with Water to Form an Acid
Hydrogen is the element that reacts with water to form an acid. When in a gaseous form, hydrogen reacts with water to form hydrohalic acids (HX), such as hydrochloric acid (HCl) and othr strong acids. Hydrochloric acid is formed when hydrogen chloride (HCl) gas dissolves in water and splits into its ions, H+ and Cl-. The resulting mixture of H+ and Cl- ions in water is what we call hydrochloric acid.

Source: tyentusa.com
Conclusion
In conclusion, acids are powerful substances that can cause serious damage if handled incorrectly. It is important to remember to always add acid to water and not the other way around when mixing the two in order to avoid splashing and heat generation. When added correctly, acids can be used safely in many different applications such as cleaning, manufacturing and cooking. There are a range of different acids availble with varying levels of concentration so it is important to be aware of which one you are using in order to ensure safety and effectiveness.
