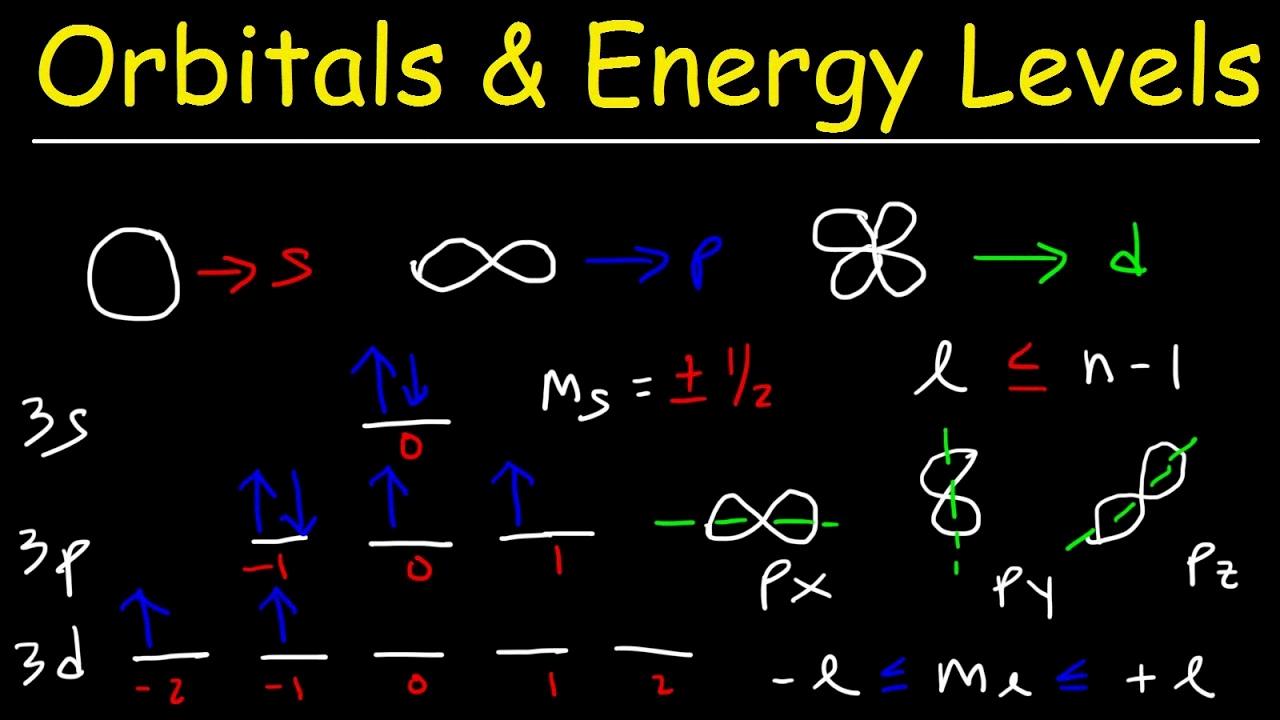
Sublevels are a way of organizing the electrons in an atom. Every atom has electrons orbiting around its nucleus, and these electrons can be organized into different shells or energy levels. Each shell is further divided into sublevels, which are labeled s, p, d, and f. The s sublevel has one orbital and can hold two electrons; the p sublevel has three orbitals and can hold six electrons; the d sublevel has five orbitals and can hold ten electrons; and the f sublevel has seven orbitals and can hold fourteen electrons.
The first period of elements (those on the far left side of the periodic table) only have one electron in their outermost shell, so they are filled with two electrons in their 1s sublevel. As more elements are added to each period of the periodic table, more shells and sublevels must be filled. For example, sodium’s first shell is filled with 2 electrons (1s), its second shell is filled with 8 electrons (2s and 2p), and its third shell is filled with 1 electron (3s).
By understanding how these shells and sublevels work, we can better understand chemical reactions on an atomic level. Knowing which elements have how many electrons in their outermost shells helps us determine reactivity levels – why some atoms bond easily while others don’t. Additionally, knowledge of these shells helps explain why certain elements form different compounds when combined together – why sodium forms NaCl (table salt) while potassium forms KCl (potassium chloride).
Chemistry sublevels may seem daunting at first glance, but once you get a grasp on them you will open up a whoe new world of atomic interactions!
Types of Sublevels
The four types of sublevels are s, p, d, and f. The s sublevel is the first and simplest, containing a single orbital that can hold up to two electrons. The p sublevel contains thee orbitals which can each hold two electrons, allowing for a maximum of six electrons. The d sublevel contains five orbitals which can each hold two electrons, allowing for a maximum of ten electrons. Finally, the f sublevel contains seven orbitals which can each hold two electrons, allowing for a maximum of fourteen electrons. Each type of sublevel has its own unique properties and characteristics which determine how it interacts with other particles.
Source: youtube.com
Understanding S and P Sublevels
The s and p sublevels are two of the four types of atomic orbitals. The s sublevel is a spherical orbital that can hold up to two electrons. It is the lowest energy orbital and is found in the first period of the periodic table. The p sublevel has three lobes, each capable of holding two electrons, and is higher in energy than the s sublevel. It can be found in the second through sixth periods of the periodic table. Both s and p orbitals are important for understanding how atoms interact with each other in chemical bonds.
What Do 1s, 2s, and 2p Represent in Chemistry?
In chemistry, 1s 2s 2p is a shorthand notation that refers to the electron configuration of an atom. Specifically, it means that the first shell (1s) of the atom is filled with two electrons, and the second shell (2s and 2p) is filled with eight electrons. This indicates that the outermost energy level of the atom is occupied by one electron. This arrangement of electrons is typical for atoms like sodium, magnesium, and aluminum.
Order of Sublevels in Electron Shells
The 4 sublevels of the electron shells, in order, are s, p, d, and f. The s sublevel is the first shell and has 1 orbital that can hold up to 2 electrons. The p sublevel is the second shell and has 3 orbitals that can hold up to 6 electrons. The d sublevel is the third shell and has 5 orbitals that can hold up to 10 electrons. Finally, the f sublevel is the fourth shell and has 7 orbitals that can hold up to 14 electrons.
Types of Orbitals
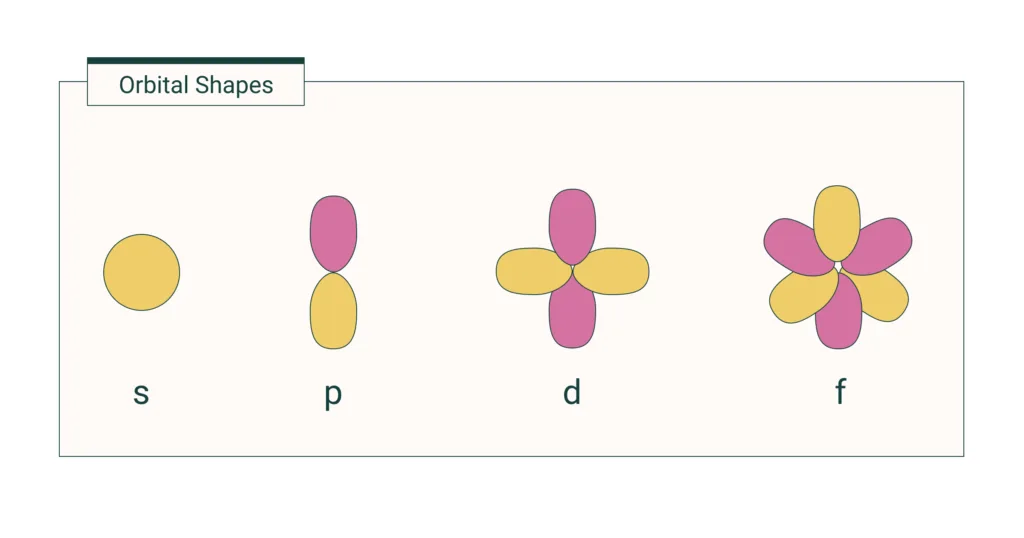
The four types of orbitals are s, p, d, and f. The s orbital is a spherical shape that can hold up to two electrons. There are three p orbitals that resemble a dumbbell shape and each have a different orientation in space; they can hold up to six electrons. The d orbital has an oblong shape and can hold up to ten electrons, while the f orbital is complex with a cloverleaf shape and can hold up to fourteen electrons. All of these different orbitals play an important role in determining the properties of atoms and molecules.
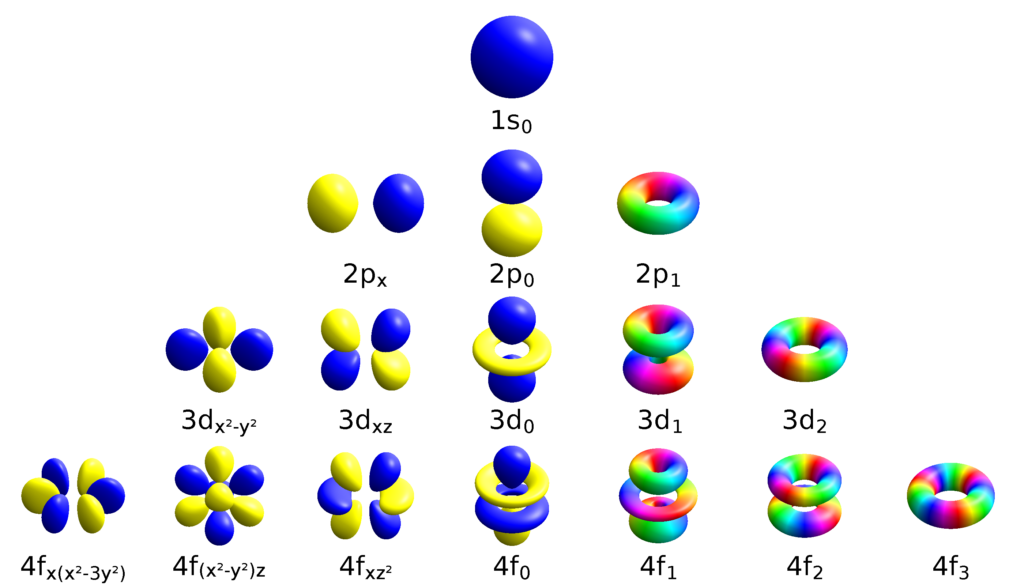
Do Sublevels of 5 Exist?
Yes, 5s sublevels do exist. The 5s sublevel is the s-orbital of the fifth energy level (or principal energy level). This means that it is the first orbital to be filled in the fifth energy level and holds two electrons. The 5s sublevel has a spherical shape, with a radius of approximately 10.9 angstroms and a diameter of 21.8 angstroms. It has one node, which is located at its center, and its angular momentum quantum number (l) is equal to 0.
The Meaning of 1s, 2s, 2p, 3s, and 3p
1s 2s 2p 3s 3p are the energy levels of electrons in an atom. The number before the letter s and p indicate the principal quantum number (n) and the letter indicates angular momentum quantum number (l).
The s-orbitals have zero angular momentum (l=0) while p-orbitals have one unit of angular momentum (l=1). The principal quantum number, n, indicates the size or distance of an orbital from the nucleus. Thus, 1s is the innermost orbital and 7p is the outermost orbital. Each energy level can contain up to two electrons with opposite spins.
The S and P Orbitals
The s/p and d orbitals are the three different types of atomic orbitals found in an atom. The s orbital is a spherical orbital with the nucleus at its centre, whereas the p orbitals are dumbbell-shaped and have two lobes pointing in opposite directions. The d orbitals are more complex, with four of the five bing cloverleaf shaped and the fifth having an elongated dumbbell shape with a doughnut around its middle. These orbitals are organized into different electron shells depending on the energy levels of each orbital. Each orbital has a specific number of electrons it can hold, which determines its overall structure and chemical properties.
The Difference Between SP and D Subshells
Yes, s and p subshells are two of the four different types of subshells in an atom. The s subshell has a spherical shape, with a capacity for holding up to two electrons. The p subshell has a dumbbell or cloverleaf-like shape, with a capacity for holding up to six electrons. The other two subshells are the d subshell, which has a slightly more complex shape and can hold up to ten electrons, and the f subshell, which is even more complex and can hold up to fourteen electrons.
The Electron Configurations of the First Four Energy Levels
The term 1s 2s 2p 3s 3p 4s refers to the electron orbital energy levels of an atom. The 1s orbital is the lowest energy level, followed by the 2s and 2p orbitals that are slightly higher in energy. The 3s and 3p orbitals are then higher in energy than the 2s and 2p orbitals, with the 4s orbital being the highest of these five. These four orbitals, known as s-orbitals, all have a spherical shape. Electrons in these orbitals have a higher probability of being found near the nucleus than those in p-orbitals, which have a dumbbell shape.
The 2-8-8-18 Rule in Chemistry
The 2 8 8 18 rule in chemistry states that the maximum number of electrons that can be held by each energy level or shell of an atom is 2, 8, 8, 18. This relates to the quantum numbers n, l and ml. The principle quantum number (n) indicates the shell number; n=1 for the first shell, n=2 for the second shell and so on. The angular momentum quantum number (l) indicates the subshell within a given shell; l=0 for s-subshells, l=1 for p-subshells, l=2 for d-subshells and so on. The magnetic quantum number (ml) indicates the paricular orbital within a given subshell; ml=-1,-2…0, 1..+2 for s-subshells and ml=-3,-2…0, 1..+3 for p-subshells.
The 2 8 8 18 rule states that in the first shell (n=1), there can be a maximum of two electrons in one s-orbital (l=0; ml=-1, 0). In the second shell (n=2), there can be a maximum of eight electrons distributed among four orbitals: two in an s-orbital (l=0; ml=-1 0) and six in three p-orbitals (l=1; ml=-3 -2 -1 0 +1 +2). In the third shell (n=3), there can be a maximum of eighteen electrons: two in an s-orbital (l=0; ml=-1 0), six in three p-orbitals (l=1; ml=-3 -2 -1 0 +1 +2) and ten in five d-orbitals (l=2; ml=-4 -3 -2 -1 0 +1 +2 +3 +4). This pattern continues up to higher shells with similar logic.
Understanding 2p Subshells
The 2p subshell is one of two subshells within the second shell of electrons. It has a maximum capacity of 6 electrons and is filled with electrons after the 2s subshell. The 2p subshell is composed of 3 orbitals, px, py, and pz, which each hold a maximum of 2 electrons. Each orbital is distinguished by its angular momentum quantum number, or l-value, which is always equal to 1 for the p orbitals. The shape of the p orbitals is that of a dumbbell with a nodal plane between the two lobes.
The First Four Orbitals
The fist four orbitals are the s, p, d, and f orbitals. The s orbital is a spherical shape and has the lowest energy of the four orbitals. It can hold up to a maximum of two electrons. The p orbital is a dumbbell shape and has higher energy than the s orbital. It can hold up to six electrons. The d orbital is an elongated shape with even higher energy than the previous two orbitals and can hold up to ten electrons. Finally, the f orbital has the highest energy of all four orbitals and is more complexly shaped than the other three. It can hold up to fourteen electrons.
What Comes After the Fourth Dimension Sublevel?
The 4d sublevel is followed by the 5s sublevel, which is located in the same row as the 4d sublevel. This means that the 5s sublevel has a lower energy than the 4d sublevel and therefore is filled first. After that, the 5p sublevel is filled. The 5p sublevel is located in the same column as the 4d sublevel, meaning that it has a higher energy than the 4d sublevel and therefore is filled after it.
Exploring the Possibility of a 4D Sublevel
Yes, there is a 4d sublevel. It is an energy sublevel, also known as an orbital, that exists in the fourth electron shell of an atom. The 4d sublevel contains five orbitals, each with a maximum capacity of two electrons with opposite spins. This means that the 4d sublevel can hold up to 10 electrons in total.
Conclusion
In conclusion, the sublevels of chemistry are an important concept to understand when studying atoms and their structure. Sublevels are organized into four types: s, p, d and f. Each of these sublevels has a specific number of orbitals that can hold a maximum of two electrons. The first shell is filled with two electrons while the second shell consists of eight electrons and the third shell has one electron. Understanding the concepts of sublevels is essential for furthering our understanding of chemistry and its applications.
