Have you ever wondered if ions are hydrophobic or hydrophilic? Well, the answer is both! It all depends on the type of ions and their charge.
Ions can be generally divided into two categories: cations (positively charged ions) and anions (negatively charged ions). Cations are typically hydrophobic, while anions are typically hydrophilic. This is because cations tend to have a stronger electrostatic repulsion between them, which makes them less likely to interact with water molecules. On the other hand, anions have weaker electrostatic repulsion and thus more likely to interact with water molecules.
In addition to their charge, the molecular structure of the ion can also affect its hydrophobicity or hydrophilicity. For example, some anions like halide ions (F-, Cl-, Br- and I-) have a higher affinity for water due to their small size and highly polarizable electrons. On the other hand, larger anions like sulfate (SO4 2-) or phosphate (PO4 3-) are more likely to be hydrophobic due to their relatively large size and non-polarizable electrons.
The physical properties of a molecule also play a role in determining its hydrophobicity or hydrophilicity. Generally speaking, molecules with higher melting points and boiling points tend to be more hydrophobic than those with lower melting points and boiling points. This is because highly ordered molecules with higher melting points require more energy to break down into smaller particles that can interact with water molecules.
Finally, functional groups present in a molecule can also influence its hydrophobicity or hydrophilicity. Common functional groups such as alcohols (OH), amines (NH2) and carboxylic acids (COOH) tend to be highly polarizable and thus increase the overall polarity of a molecule making it more likely for it to interact with water molecules. On the other hand, non-polar functional groups such as alkenes (C=C) tend to decrease overall polarity making a molecule less likely to interact with water molecules.
Overall, whether or not an ion is considered hydrophobic or hydrophilic depends on several factors such as its charge, molecular size, physical properties and presence of functional groups. Knowing this information can help us make better predictions about how different materials may interact with each other in solution.
The Hydrophilicity of Charged Ions and Polar Molecules
Yes, charged ions and polar molecules are both hydrophilic. Charged ions, like sodium, chloride and potassium, have an overall charge that makes them attracted to water molecules. Polar molecules are made up of atoms with slightly different charges, which creates regions of partial positive or negative charge on the molecule. This creates a dipole moment, which makes the molecule attracted to water molecules. As a result, both charged ions and polar molecules are hydrophilic and dissolve easily in water.
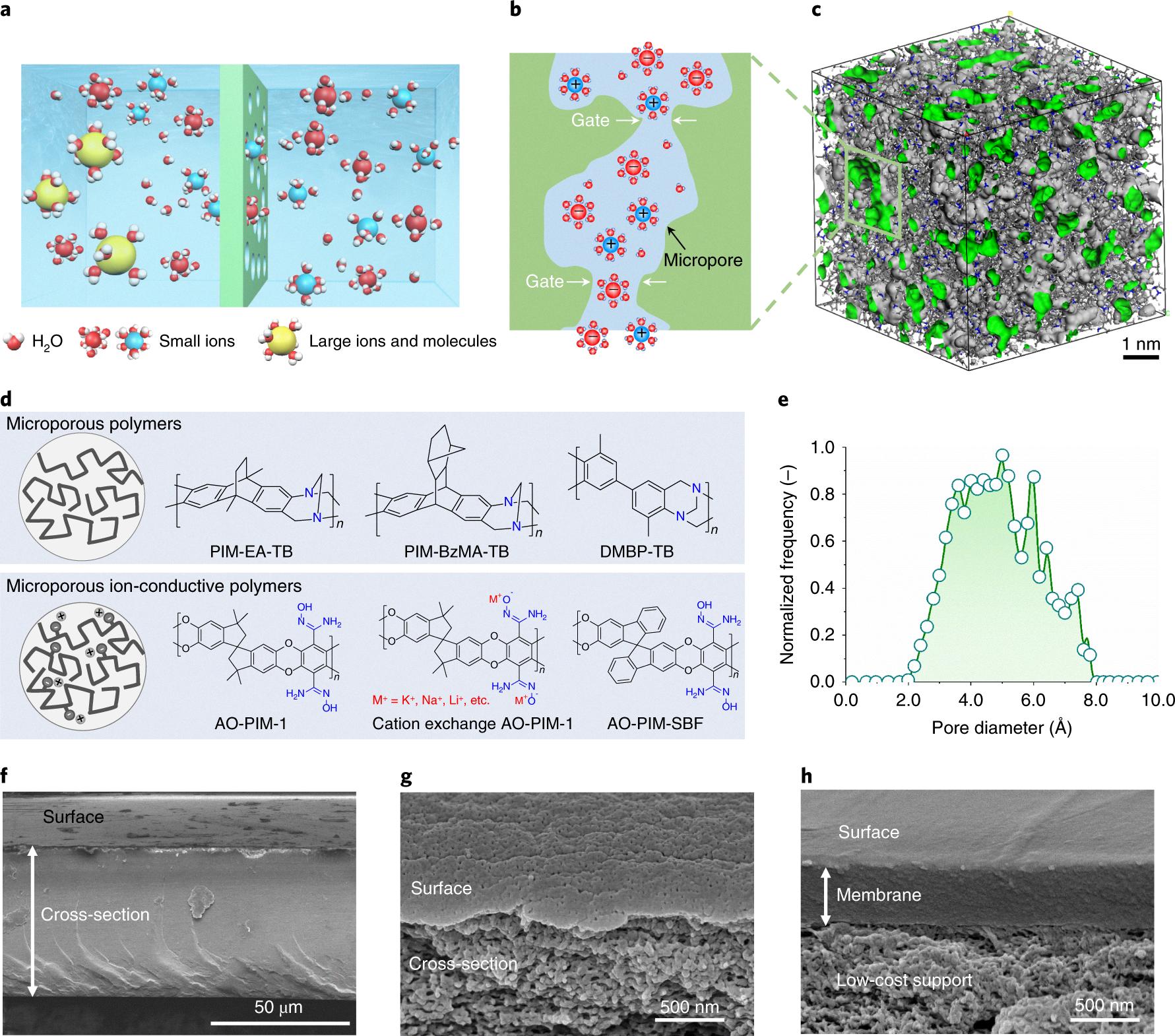
Source: nature.com
Are Anions Hydrophobic?
Yes, anions are generally considered to be hydrophobic. This is because they are typically soft, weakly hydrated and have relatively weak Coulombic forces. Anions have a tendency to interact more strongly with organic solvents than with water molecules, which makes them less likely to dissolve in water. As such, they tend to stick together and form clusters that repel the surrounding water molecules. This behavior makes them more hydrophobic than other ions like cations.
Are Ions Hydrophilic Substances?
No, hydrophilic substances are not necessarily ions. While many hydrophilic substances contain ionic (charged) groups, they can also contain polar molecules and nonpolar molecules with no charge. For example, some sugars and amino acids are considered to be hydrophilic because of their large surface area and ability to interact with water molecules, but they do not contain any ions.
Types of Hydrophilic Bonds
Hydrophilic molecules are molecules that have a strong affinity for water and interact strongly with it. Such molecules contain either polar covalent bonds or ionic bonds. Polar covalent bonds involve the sharing of electrons between two atoms, with one atom having a stronger attraction for the electrons than the other, resulting in an uneven distribution of charge wihin the bond. This creates a molecule with a slightly negative side and a slightly positive side, making it attractive to water molecules, which are also polar. Ionic bonds involve the transfer of electrons from one atom to another, creating two ions with opposite charges, which then attract each other strongly due to electrostatic forces. These charged ions interact strongly with water molecules as they are attracted to each other’s opposite charges. Therefore, both polar covalent bonds and ionic bonds create hydrophilic molecules.
Can Ions Be Hydrophobic?
No, ions cannot be hydrophobic. Hydrophobic molecules are those that contain non-polar covalent bonds, meaning that the electrons within the molecules are shared equally between the atoms. Ions, on the other hand, are either positively charged (cations) or negatively charged (anions). This charge causes them to be attracted to water molecules and thus makes them hydrophilic (water-loving).

Are Cations Hydrophobic?
Yes, a cation is hydrophobic. Cations are positively charged particles that, due to their electrostatic charge, repel water molecules and oter polar molecules. This results in a hydrophobic character which can be observed when cations are placed in aqueous solutions. Additionally, the hydration shells of cations can further destabilize adsorbed hydroxide and adsorbed water on the surface of certain electrodes such as platinum electrodes. This hydrophobicity is beneficial for the oxygen reduction reaction at the electrode interface as it prevents poisoning species from being adsorbed onto the surface of the electrode.
Solubility of Anions in Water
Anions, which are negatively-charged ions, can be either soluble or insoluble in water. Most halides (chloride, bromide and iodide) are soluble in water, except with magnesium and silver cations (Mg2+ and Ag+). Most hydroxides (OH–) are insoluble, but alkali metal hydroxides such as sodium hydroxide (NaOH) and potassium hydroxide (KOH) are soluble. Carbonates (CO32–), sulfates (SO42–), and phosphates (PO43–) usually have low solubility in water; however, the singly charged versions of thse anions—hydrogen carbonate (HCO3–), hydrogen sulfate (HSO4–), and dihydrogen phosphate/hydrogen phosphate (H2PO4–)—are usually more soluble.
Is Ion Hydrophobic or Hydrophilic?
Ions are hydrophilic, meaning they are attracted to water. This is because ions have either a positive or negative charge, which is attracted to the partial positive and partial negative charges of water molecules (hydrogen and oxygen, respectively). Non-polar, non-ionic molecules are hydrophobic because there is nothing for the partial negative oxygen of water and/or the partial positive hydrogen of water to be attracted to.
Types of Hydrophobic Substances
Hydrophobic substances are composed of molecules that have non-polar characteristics, meaning they lack an overall charge. This makes them repellent to water and other polar molecules, and instead attract other neutral molecules and non-polar solvents. Common hydrophobic molecules include alkanes, oils, fats, waxes, and hydrocarbons. Waxes are a special type of hydrophobic substance that can form a protective coating on surfaces due to their hydrophobicity. Alkanes are an important group of hydrophobic substances that consist of single bonds betwen carbon atoms; long-chain fatty acids are examples of this group. Oils and fats are also composed of long-chain fatty acids but with double bonds between the carbon atoms. Hydrocarbons are another type of hydrophobic molecule that contain only hydrogen and carbon atoms in their structure; they include alkanes, alkenes, and aromatics.
The Properties of Hydrophilic and Hydrophobic Substances
An amphipathic molecule is a type of molecule that has both hydrophilic and hydrophobic components. Hydrophilic means that the molecule attracts water molecules and is soluble in water. Hydrophobic means that the molecule repels water and is insoluble in water. These two components of an amphipathic molecule work together to form a structure that can interact with both aqueous (water-based) and non-aqueous (non-water based) environments. Examples of amphipathic molecules include lipids such as phospholipids, proteins, detergents, and surfactants.
Identifying Hydrophobic and Hydrophilic Parts
The phospholipid bilayer that makes up the cell membrane is composed of two parts – the head and the tail. The head, which is located on the outer side of the membrane, is hydrophilic (water-loving), whie the tail, which is located on the inner side of the membrane, is hydrophobic (water-fearing). The hydrophobic tails are arranged in such a way to create a barrier that prevents certain molecules from passing through. This allows cells to control what enters and leaves them, keeping their internal environment stable.
Common Examples of Hydrophobic Elements
Hydrophobic substances are substances that do not mix with or dissolve in water. These substances are composed of molecules that have a strong affinity for one another and repel water molecules. The most common hydrophobic elements include carbon, hydrogen, nitrogen, and sulfur. Other examples of hydrophobic elements include fluorine, chlorine, bromine, iodine, phosphorus, and silicon. Hydrophobic molecules have non-polar covalent bonds between their atoms and lack any areas of partial positive or negative charge. This means that they cannot form hydrogen bonds with water molecules so they tend to repel them instead.
What Are Examples of Hydrophilic Substances?
A hydrophilic molecule is one that has an affinity for water and other polar solvents. It usually consists of a polar head group and a non-polar tail, which makes it capable of forming hydrogen bonds with water molecules. In addition, the molecule may have positively or negatively charged groups on its surface that can interact with the charges in water molecules. This gives hydrophilic molecules a strong tendency to dissolve in water and other polar solvents, making them soluble in these environments. Examples of hydrophilic molecules include sugar, amino acids, salts, and proteins.
Conclusion
In conclusion, it is important to note that ions can be either hydrophobic or hydrophilic depending on their chemical structure. While ionic bonds are usually found in hydrophilic molecules, polar covalent bonds can also create molecules that are hydrophilic. Generally, if a molecule has areas where thee is a partial positive or negative charge, then it is considered to be hydrophilic. Additionally, common functional groups such as oxygen or nitrogen atoms can increase the hydrophilicity of a molecule. Therefore, understanding the chemical structure of an ion can help determine whether it is hydrophobic or hydrophilic.
