In the realm of chemistry, bonds between atoms play a crucial role in determining the properties and behavior of substances. One such bond is the ionic bond, which forms between atoms of a metal and a nonmetal. Let’s delve into the fascinating world of ionic bonding and explore what occurs during this process.
An ionic bond is essentially a force of attraction that holds together oppositely charged ions. It arises when atoms of a metal transfer electrons to atoms of a nonmetal. This electron transfer leads to the formation of ions, which are charged particles. The metal atom loses electrons to become a positively charged cation, while the nonmetal atom accepts these electrons to become a negatively charged anion. The resulting attraction between the oppositely charged ions forms the ionic bond.
To illustrate this further, let’s consider some examples of ionic bonds. Sodium chloride (NaCl), sodium bromide (NaBr), and sodium fluoride (NaF) are classic examples of compounds formed through ionic bonding. In each case, the sodium atom donates an electron to the respective nonmetal atom, resulting in the formation of positively charged sodium ions (Na+) and negatively charged chloride (Cl-), bromide (Br-), or fluoride (F-) ions. The resulting electrostatic attraction between these oppositely charged ions forms the ionic bond.
One notable characteristic of ionic compounds is their high melting points. This is due to the strong electrostatic forces of attraction between the ions in the crystal lattice structure. These forces require a significant amount of energy to overcome, resulting in the high melting point of ionic compounds.
Additionally, ionic compounds tend to be hard and brittle. This is because the arrangement of ions in the crystal lattice is rigid and orderly. When a force is applied, the layers of ions can easily shift, causing the crystal to fracture rather than deform. This brittleness is what gives ionic compounds their characteristic property.
Another important aspect of ionic compounds is their behavior in water. When an ionic compound is dissolved in water, it dissociates into its constituent ions. The water molecules surround the individual ions, separating them from one another. This process, known as dissociation, allows ionic compounds to conduct electricity when dissolved in water, as the ions are free to move and carry an electric charge.
Ionic bonding is a fascinating concept that involves the complete transfer of valence electrons between atoms. This transfer results in the formation of oppositely charged ions, which then attract each other to form an ionic bond. The resulting compounds have high melting points, are hard and brittle, and dissociate into ions when dissolved in water. The examples provided, such as sodium chloride, sodium bromide, and sodium fluoride, serve as excellent illustrations of the occurrence of ionic bonding.
Remember, understanding the forces that bind atoms together is crucial in comprehending the properties and behavior of different substances. Ionic bonding is just one of the many wonders of chemistry that shapes the world around us.
What Occurs In An Ionic Bond?
In an ionic bond, oppositely charged ions are attracted to each other and held together by the force of attraction. This bond is formed when electrons are transferred from atoms of a metal to atoms of a nonmetal. The metal atom loses one or more electrons to become a positively charged ion, while the nonmetal atom gains these electrons to become a negatively charged ion.
In more explicit terms, the process of forming an ionic bond involves the following steps:
1. Metal atoms, which tend to have few valence electrons, will donate one or more of these electrons to nonmetal atoms.
2. As a result, the metal atoms become positively charged ions, known as cations, since they have more protons than electrons.
3. Conversely, the nonmetal atoms gain these donated electrons and become negatively charged ions, known as anions, since they now have more electrons than protons.
4. The opposite charges of the cations and anions attract each other and create a strong electrostatic force that holds them together, forming an ionic bond.
It is important to note that in an ionic compound, such as table salt (sodium chloride), the ions are arranged in a regular, repeating pattern known as a crystal lattice. This arrangement allows for the efficient packing of ions in a three-dimensional structure.
An ionic bond occurs when electrons are transferred between atoms of a metal and a nonmetal, resulting in the formation of oppositely charged ions that are held together by the attractive force between them. This bond leads to the formation of ionic compounds, which have a crystalline structure instead of individual molecules.
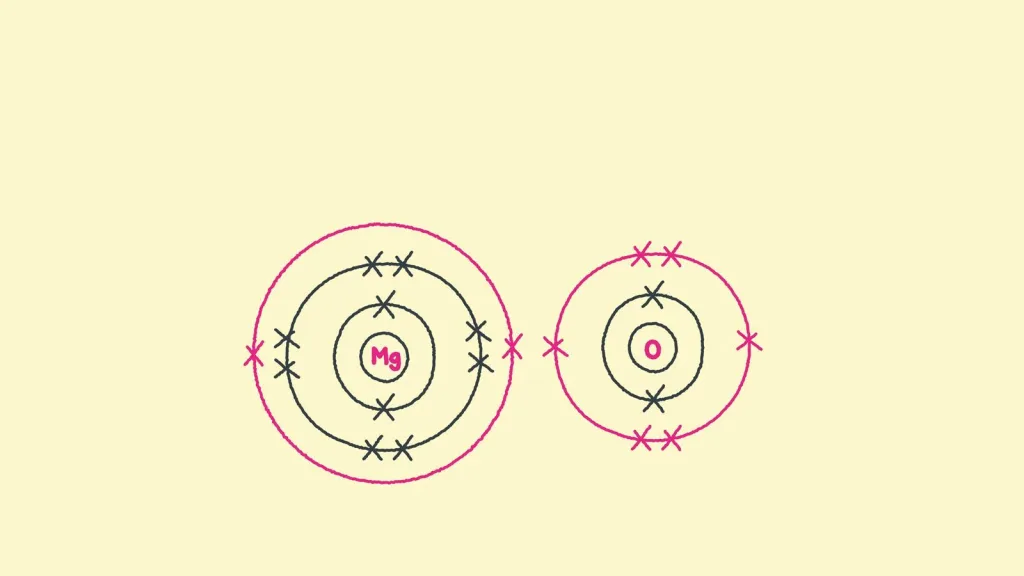
Which Of The Following Takes Place In An Ionic Bond?
In an ionic bond, the following processes take place:
1. Transfer of electrons: Ionic bonding involves the complete transfer of valence electrons between atoms. Valence electrons are the outermost electrons in an atom’s electron shell. In this process, the metal atom donates one or more electrons to the nonmetal atom.
2. Formation of ions: As a result of the electron transfer, the metal atom loses electrons and becomes a positively charged ion called a cation. The nonmetal atom gains electrons and becomes a negatively charged ion called an anion. These oppositely charged ions are held together by electrostatic forces of attraction.
3. Electrostatic attraction: The cation and anion are attracted to each other due to the opposite charges. The positive charge of the cation is attracted to the negative charge of the anion, creating a strong electrostatic force that holds the two ions together.
4. Stability: Ionic bonding occurs between elements with significantly different electronegativity values. Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. The large difference in electronegativity results in a complete transfer of electrons, leading to the formation of stable ions.
What Are 3 Examples Of An Ionic Bond?
Three examples of ionic bonds are:
1. Sodium chloride (NaCl): Sodium chloride, commonly known as table salt, is a classic example of an ionic bond. It is formed when sodium (Na) donates an electron to chlorine (Cl). Sodium becomes a positively charged ion (Na+) and chlorine becomes a negatively charged ion (Cl-). The resulting electrostatic attraction between the positive and negative ions creates the ionic bond in NaCl.
2. Calcium carbonate (CaCO3): Calcium carbonate is a compound found in many natural substances, such as limestone, marble, and shells. In this compound, calcium (Ca) donates two electrons to each of the three oxygen atoms (O) in the carbonate ion (CO3^2-). The resulting calcium ion (Ca^2+) and carbonate ion (CO3^2-) are held together by ionic bonds.
3. Magnesium oxide (MgO): Magnesium oxide is formed when magnesium (Mg) transfers two electrons to oxygen (O). The magnesium atom loses two electrons to become a positively charged ion (Mg^2+), while the oxygen atom gains two electrons to become a negatively charged ion (O^2-). The attraction between the oppositely charged ions creates the ionic bond in magnesium oxide.
Ionic bonds occur when there is a complete transfer of electrons from one atom to another, resulting in the formation of positively and negatively charged ions that are held together by electrostatic attractions.
What Are 3 Characteristics Of An Ionic Bond?
– Ionic compounds have high melting points: Ionic bonds are formed between ions with opposite charges. These strong electrostatic forces of attraction require a large amount of energy to break the bond and melt the compound. As a result, ionic compounds generally have high melting points.
– Ionic compounds are hard and brittle: The strong electrostatic forces in an ionic bond also make the compound hard and brittle. When a force is applied to an ionic compound, the ions are forced out of their regular lattice arrangement, causing the compound to shatter.
– Ionic compounds dissociate into ions when dissolved in water: When an ionic compound is dissolved in water, the water molecules surround the ions and pull them apart, breaking the ionic bond. This process is called dissociation, and it results in the formation of separate positive and negative ions in the solution. This property of ionic compounds allows them to conduct electricity when dissolved in water.
Conclusion
An ionic bond is a strong force of attraction that forms between oppositely charged ions. This bond occurs when electrons are transferred from atoms of a metal to atoms of a nonmetal, resulting in the formation of positively charged cations and negatively charged anions. Ionic compounds, such as sodium chloride, sodium bromide, and sodium fluoride, have high melting points and are hard and brittle. When dissolved in water, these compounds dissociate into ions. Understanding the nature of ionic bonding is crucial in explaining the properties and behavior of ionic compounds.
