The periodic table is a useful tool for understanding the properties of elements. It is a chart that organizes the chemical elements based on their atomic structure and properties. One group of elements that can be found in the periodic table are the semimetals, also known as metalloids.
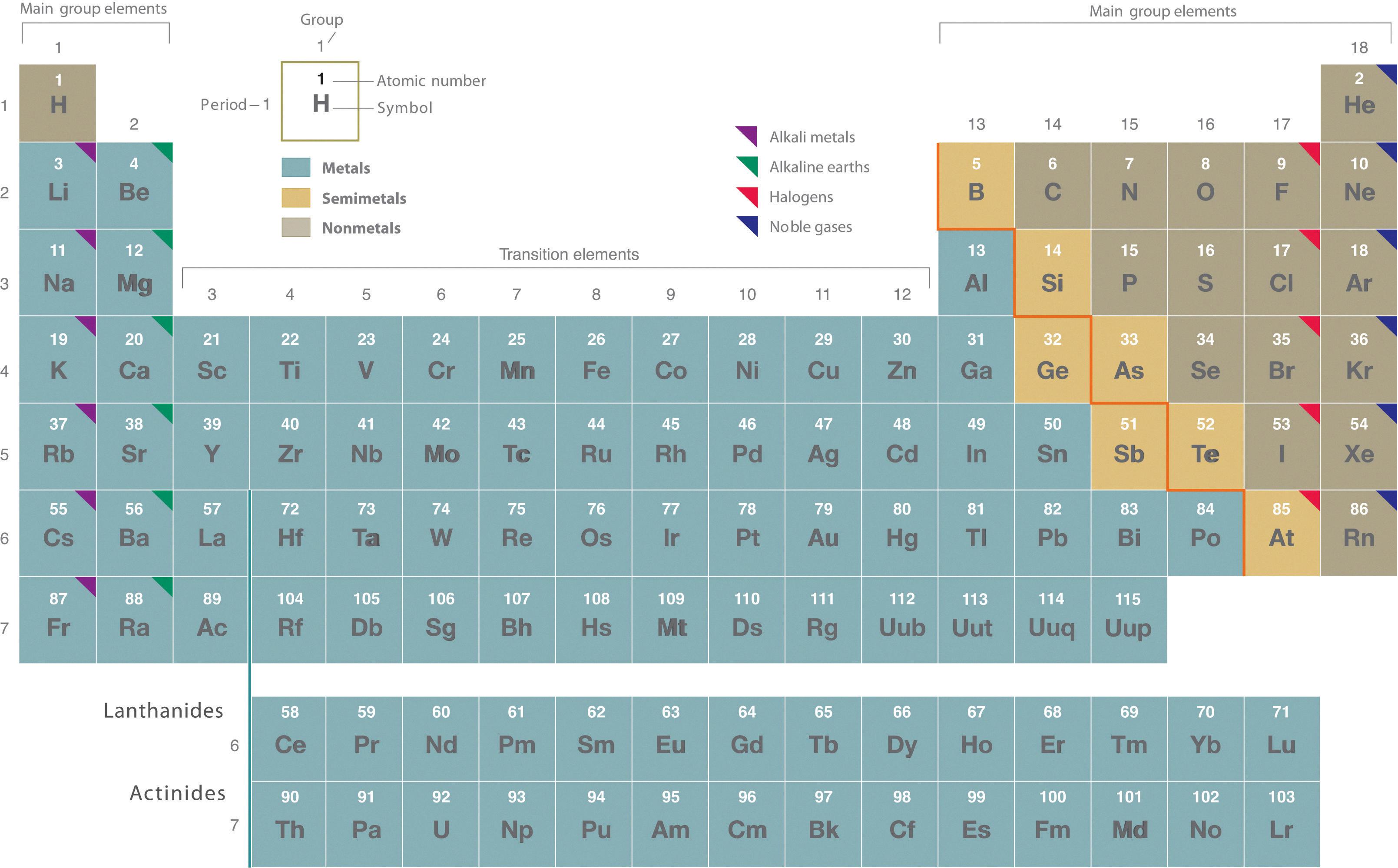
Semimetals are elements that have properties that are intermediate between metals and nonmetals. They are located in a diagonal line that separates the metals from the nonmetals. There are eight semimetals in total: boron (B), silicon (Si), germanium (Ge), arsenic (As), antimony (Sb), tellurium (Te), polonium (Po), and astatine (At).
One of the defining characteristics of semimetals is their electrical conductivity. Unlike metals, which are good conductors of electricity, and nonmetals, which are poor conductors, semimetals have a variable level of conductivity. This makes them useful for a variety of applications in electronics and other industries.
Another property of semimetals is their ability to form alloys. An alloy is a material made up of two or more metals or a metal and a nonmetal. Semimetals can be used to create alloys that have specific properties, such as increased strength or resistance to corrosion.
Semimetals are also important in the field of semiconductors. A semiconductor is a material that can conduct electricity under certain conditions, but not under others. Semimetals like silicon and germanium are commonly used in the production of computer chips and other electronic devices.
In addition to their practical uses, semimetals also have important roles in the natural world. For example, arsenic can be found in some minerals and is used in the production of pesticides and other chemicals. Boron is an essential nutrient for plants and is used in the production of fertilizers.
The semimetals are a diverse group of elements that have properties that are intermediate between metals and nonmetals. They have a variety of practical uses in industry and technology, as well as important roles in the natural world. By understanding the properties of semimetals, we can better appreciate the complexity and diversity of the periodic table.
What Are The Semimetals On The Periodic Table?
The semimetals, also known as metalloids, are a group of elements that are located between the metals and the non-metals on the periodic table. These elements include Boron in Group 13, Silicon and Germanium in Group 14, Arsenic and Antimony in Group 15, Tellurium and Polonium in Group 16, and Astatine in Group 17.
Semimetals have properties of both metals and non-metals, making them unique in teir chemical and physical characteristics. They can conduct electricity, but not as well as metals, and can also act as insulators under certain conditions. Semimetals also have varying levels of reactivity and melting points, with some being brittle solids and others being shiny, metallic-looking solids.
The semimetals on the periodic table are Boron, Silicon, Germanium, Arsenic, Antimony, Tellurium, Polonium, and Astatine.
What Are The 8 Semi Metals?
The 8 semi-metals are also known as metalloids. They are elements that exhibit properties of both metals and non-metals. The 8 semi-metals are antimony (Sb), germanium (Ge), silicon (Si), arsenic (As), tellurium (Te), polonium (Po), boron (B), and astatine (At). These elements are located along the zigzag line on the periodic table between the metals and non-metals. They have unique electronic configurations and exhibit properties such as semi-conductivity, brittleness, and metal-like luster. The metalloids have important applications in various fields such as electronics, optoelectronics, and metallurgy.
How Many Semimetals Are On The Periodic Table?
There are 17 semimetals, also known as metalloids, located on the periodic table. These elements possess properties of both metals and nonmetals, such as intermediate conductivity, and are commonly used in the production of semiconductors. Examples of semimetals include boron, silicon, germanium, arsenic, antimony, and tellurium.
What Are Called Semimetals?
Semimetals, also known as metalloids, are a group of chemical elements that exhibit properties of both metals and nonmetals. They are located in the periodic table between the metals and nonmetals, with their properties lying between those of the two categories. Semimetals have intermediate conductivity, which means that they conduct electricity better than nonmetals but not as well as metals. They also have varying degrees of metallic luster and are typically brittle. Some common examples of semimetals include silicon, arsenic, germanium, antimony, and tellurium. Semimetals are widely used in modern technology, particularly in the semiconductor industry, where they are used to make computer chips and oter electronic devices.
Conclusion
The semimetals, also known as metalloids, occupy a unique position in the periodic table between the metals and nonmetals. This group includes elements such as boron, silicon, arsenic, and tellurium, which possess both metallic and nonmetallic properties. Semimetals have a variety of applications in technology and industry, particulrly in the production of semiconductors and electronic devices. While they share some similarities with both metals and nonmetals, semimetals have distinct properties that make them invaluable in many fields. As our understanding of these fascinating elements continues to grow, it is clear that they will continue to play a vital role in shaping the world around us.
