Ammonia, also known as NH3, is a compound that is commonly used in many industrial and household applications. It is a colorless gas that has a pungent odor and is highly soluble in water. In this article, we will explore the properties of NH3 and why it is soluble in water.
The molecular structure of NH3 consists of one nitrogen atom and thre hydrogen atoms. The nitrogen atom has a lone pair of electrons, which makes NH3 a polar molecule. This polarity allows NH3 to form hydrogen bonds with water molecules, which is why it is highly soluble in water.
When NH3 dissolves in water, it undergoes a process called ionization. This means that NH3 molecules react with water molecules to form ammonium ions (NH4+) and hydroxide ions (OH-). The equation for this reaction is as follows:
NH3 + H2O → NH4+ + OH-
This reaction is reversible, which means that ammonium ions and hydroxide ions can react to form NH3 and water molecules. This process is known as dissociation.
The solubility of NH3 in water depends on several factors, such as temperature and pressure. At room temperature and standard pressure, NH3 is highly soluble in water, with a solubility of about 30 g/L. However, as the temperature increases, the solubility of NH3 decreases, which means that it is less soluble in hot water than in cold water.
The solubility of NH3 is also affected by the presence of other substances in water. For example, NH3 reacts with acids to form ammonium salts, which can reduce its solubility in water. Similarly, NH3 can react with certain metals to form metal amides, which can also affect its solubility in water.
NH3 is a polar molecule that is highly soluble in water due to its ability to form hydrogen bonds with water molecules. When NH3 dissolves in water, it undergoes ionization to form ammonium ions and hydroxide ions. The solubility of NH3 in water depends on various factors, such as temperature and the presence of other substances in water. Understanding the properties of NH3 and its solubility in water is important in many industrial and scientific applications.
Why Is NH3 Not Soluble In Water?
NH3 is a polar molecule due to the presence of a lone pair of electrons on the nitrogen atom. However, despite being polar, NH3 is not very soluble in water. This is because it is not capable of forming hydrogen bonds with water molecules to the same extent as other polar molecules such as H2O itself. Hydrogen bonds occur between molecules containing a hydrogen atom bonded to an electronegative element such as oxygen or nitrogen, and another electronegative element with a lone pair of electrons. In the case of NH3, altough it does have a hydrogen atom bonded to nitrogen, the lone pair of electrons is already occupied, making it less likely to form hydrogen bonds with water molecules. Furthermore, the size of the NH3 molecule is larger than that of a water molecule, which makes it more difficult for NH3 molecules to fit into the spaces between water molecules and become dissolved. Therefore, NH3 is only slightly soluble in water and forms a weak alkaline solution when dissolved.
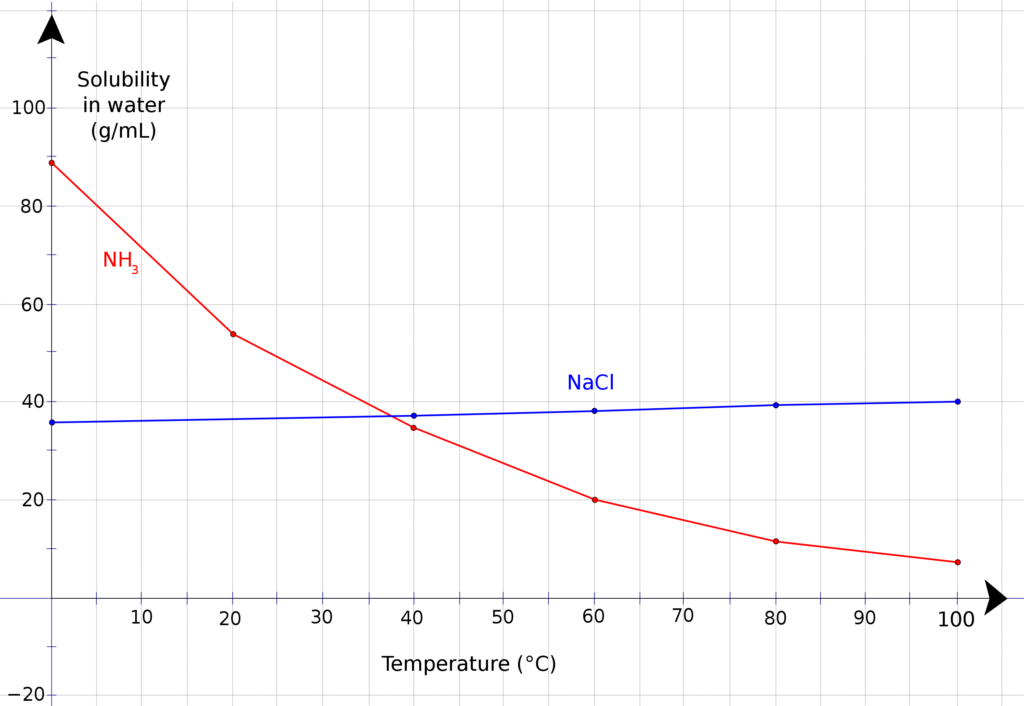
Is NH 3 Soluble In Water?
NH3 (ammonia) is highly soluble in water. This is because of the polar nature of NH3. The nitrogen atom in NH3 has a lone pair of electrons, which creates a partial negative charge on the nitrogen atom and a partial positive charge on the hydrogen atoms. This polarity alows NH3 to form hydrogen bonds with water molecules, which makes it soluble in water.
Furthermore, NH3 is a small molecule, and its size allows it to easily fit into the spaces between water molecules. This increases the solubility of NH3 in water.
NH3 is highly soluble in water due to its polarity and small molecular size.
Is NH3 Soluble In Water Or Oil?
NH3 (ammonia) is a polar compound and is soluble in water. This is due to the fact that ammonia can form hydrogen bonds with the water molecules. The nitrogen atom in ammonia has a lone pair of electrons, which can hydrogen bond with the hydrogen atoms of water. Similarly, the hydrogen atoms in ammonia can form hydrogen bonds with the oxygen atom of water. These hydrogen bonds make ammonia soluble in water.
On the other hand, oil is a non-polar substance. It does not have any charge separation, and its molecules do not form hydrogen bonds. Ammonia is not soluble in oil becuse of the difference in their polarities. The non-polar nature of oil does not allow ammonia to dissolve in it.
NH3 is soluble in water because of its polar nature, but it is not soluble in oil due to the non-polar nature of oil.
Conclusion
NH3 or ammonia is a polar compound that is highly soluble in water due to its ability to form hydrogen bonds with water molecules. The lone pair of electrons on the nitrogen atom plays a crucial role in making it a polar substance. When NH3 dissolves in water, ionization occurs, and it forms NH4OH. Due to its polar nature and ability to form hydrogen bonds, ammonia finds various applications in industries such as agriculture, refrigeration, and cleaning products. Its unique chemical properties make it an essential compound in our daily lives.
