Photoelectric effect is a fascinating phenomenon that has been studied by scientists for over a century. The effect is observed when a metal surface is exposed to light of a certain frequency, and electrons are emitted from the surface. The energy of the emitted electrons, also known as photoelectrons, is dependent on the frequency of the incident light and the material of the surface. In this article, we will take a closer look at the formula for the maximum kinetic energy of photoelectrons and the factors that affect it.
The maximum kinetic energy of a photoelectron is gven by the formula:
K.E. = hf – Φ
Where K.E. is the kinetic energy of the photoelectron, h is Planck’s constant, f is the frequency of the incident light, and Φ is the work function of the metal. The work function is the minimum energy required to remove an electron from the surface of the metal.
The formula tells us that the maximum kinetic energy of a photoelectron is equal to the energy of a photon minus the work function. This means that if the energy of the photon is less than the work function, no electrons will be emitted from the surface. The maximum kinetic energy is reached when the energy of the photon is equal to the work function.
The maximum kinetic energy of photoelectrons is affected by two main factors: the frequency of the incident radiation and the material on the surface.
1. Frequency of the Incident Radiation
The energy of a photon is directly proportional to its frequency. This means that as the frequency of the incident light increases, the energy of the photons also increases. As a result, the maximum kinetic energy of the emitted electrons also increases. The relationship between the frequency of the incident radiation and the maximum kinetic energy of photoelectrons is shown in the graph below:
(Image source: https://www.boundless.com/chemistry/textbooks/boundless-chemistry-textbook/quantum-theory-7/the-photoelectric-effect-69/photoelectric-effect-definition-289-3647/)
As we can see from the graph, the maximum kinetic energy of the electrons increases linearly with the frequency of the incident radiation. However, there is a minimum frequency, called the threshold frequency, below which no electrons are emitted.
2. Material on the Surface
The work function of a metal depends on the material of the surface. Different materials have different work functions, which means that the energy required to eject an electron from the surface varies. Materials with lower work functions require less energy to remove an electron, which means that the maximum kinetic energy of the emitted electrons is higher.
The maximum kinetic energy of photoelectrons is determined by the frequency of the incident radiation and the material on the surface. The formula for the maximum kinetic energy of photoelectrons is K.E. = hf – Φ, where h is Planck’s constant, f is the frequency of the incident light, and Φ is the work function of the metal. Understanding the factors that affect the maximum kinetic energy of photoelectrons is important in many areas of science and technology, including solar cells, photo detectors, and electron microscopy.
Calculating Maximum Energy
The formula for maximum energy of an emitted electron is given by subtracting the work function of the metal from the energy of a photon with frequency f. Mathematically, this can be expressed as:
Max Energy = E – W
Where E is the energy of a photon with frequency f, given by the equation E = hf, h being Planck’s constant, and W is the work function of the metal.
To furthr clarify, the work function is the minimum amount of energy required to remove an electron from the surface of a metal. It is a characteristic property of the metal and depends on factors such as the atomic structure and the chemical composition of the metal.
The formula can be used to calculate the maximum energy of emitted electrons in various physical phenomena, such as the photoelectric effect and the Compton effect. It is important to note that the maximum energy is not always equal to the kinetic energy of the emitted electrons, as some of the energy may be lost in the form of heat or other radiation.
Source: freepik.com
Finding the Maximum Kinetic Energy of an Object
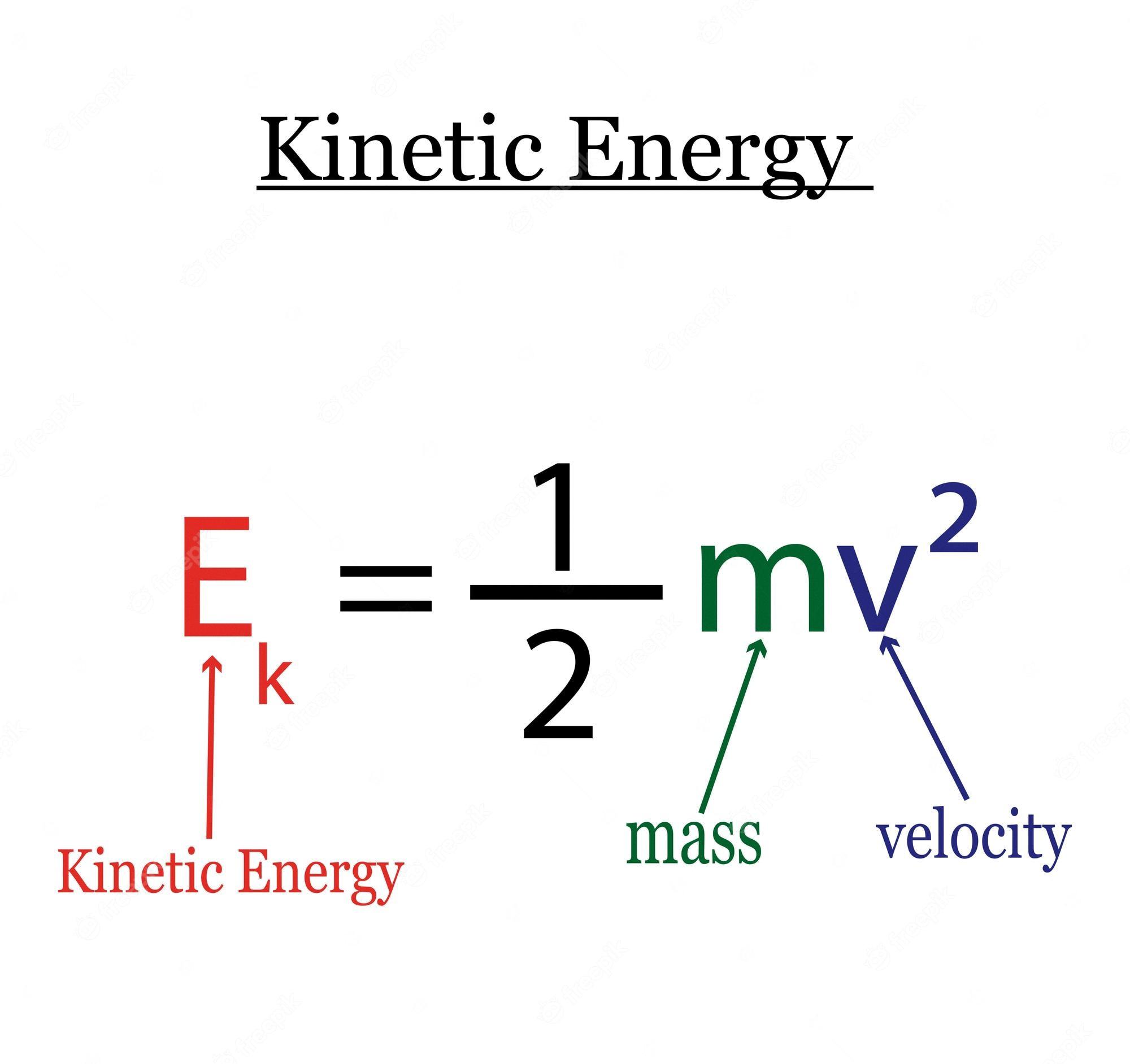
To find the maximum kinetic energy of an object, you need to know its mass and velocity. Kinetic energy is described by the followig formula: K.E. = 1/2 m v2, where K.E. stands for kinetic energy, m represents the mass of the object, and v is its velocity.
To determine the maximum kinetic energy of an object, you need to identify the maximum velocity it can achieve. This can be done by analyzing the forces acting on the object and determining the maximum acceleration it can experience. Once you have determined the maximum velocity, you can calculate the maximum kinetic energy using the formula mentioned earlier.
It is important to note that the kinetic energy of an object is directly proportional to its mass and the square of its velocity. This means that increasing either the mass or velocity of an object will result in an increase in its kinetic energy. Therefore, it is essential to consider both factors when calculating the maximum kinetic energy of an object.
In summary, to find the maximum kinetic energy of an object, you need to:
1. Determine the maximum velocity the object can achieve
2. Calculate the kinetic energy using the formula K.E. = 1/2 m v2
3. Remember that the kinetic energy is directly proportional to the mass and square of velocity of the object.
Kinetic Energy of an Electron Formula
The formula for the kinetic energy of an electron is givn by the expression:
K.E. = 1/2 mv²
Where K.E. stands for kinetic energy, m represents the mass of the electron, and v represents its velocity. This formula is used to calculate the amount of energy possessed by an electron in motion due to its velocity.
It is important to note that the mass of an electron is very small, approximately 9.11 × 10⁻³¹ kg, which means that its kinetic energy is also quite small. However, in the context of atomic and subatomic particles, even small amounts of kinetic energy can have significant effects.
It is also worth mentioning that the kinetic energy of an electron in an atom is related to its orbital radius and the electrostatic force between the electron and the nucleus. In fact, the energy of an electron in a specific energy level in an atom can be calculated using the formula:
E = (-13.6 eV) / n²
Where E is the energy of the electron, n is the energy level, and eV stands for electron volts. This formula is derived from the principles of quantum mechanics and helps to explain the behavior of electrons in atoms.
The formula for the kinetic energy of an electron is K.E. = 1/2 mv², and it is related to the electron’s velocity and mass. Additionally, the energy of an electron in an atom can be calculated using the formula E = (-13.6 eV) / n², which is derived from quantum mechanics principles.
Maximum Kinetic Energy in Photoelectric Effect
The photoelectric effect is a phenomenon where electrons are emitted from a material’s surface when it is exposed to electromagnetic radiation, such as light. The maximum kinetic energy of photoelectrons is determined by two factors: the frequency of the incident radiation and the material on the surface.
The frequency of the incident radiation determines the energy of the photons that are emitted. The energy of a photon is directly proportional to its frequency, which means that higher frequency radiation carries more energy. When a photon strikes a material’s surface, it can transfer its energy to an electron, causing it to be emitted from the material. The maximum kinetic energy of the emitted electron is equal to the energy of the absorbed photon minus the work function of the material.
The work function is the minimum amount of energy required to remove an electron from the material’s surface. This value is different for different materials, and it depends on the properties of the material, such as its chemical composition and crystal structure.
When the frequency of the incident radiation is below a certain threshold, no electrons are emitted from the material’s surface, regrdless of the intensity of the radiation. This is because the energy of the photons is not sufficient to overcome the work function of the material. However, once the frequency of the incident radiation exceeds the threshold, electrons are emitted, and their kinetic energy increases linearly with the frequency of the radiation.
The maximum kinetic energy of photoelectrons is determined by the frequency of the incident radiation and the work function of the material. Higher frequency radiation carries more energy, and the work function is the minimum amount of energy required to remove an electron from the material’s surface.
Conclusion
The maximum kinetic energy of a photoelectron is determined by two main factors: the frequency of the incident radiation and the material of the surface. The energy of a photon for a given frequency minus the energy required to eject an electron from the metal’s surface gives the maximum energy of an emitted electron. In the case of the first Bohr’s orbit, the kinetic energy is 13.6eV.
The relationship between electron energy and frequency is linear above the threshold, as shown in the graph. Therefore, increasing the frequency of the incident radiation will increase the maximum kinetic energy of the photoelectrons.
The material of the surface also plays a crucial role in determining the maximum kinetic energy of photoelectrons. The work function of a metal, which is the energy required to eject an electron from its surface, varies for differnt materials. Metals with a lower work function will require less energy to eject an electron, resulting in a higher maximum kinetic energy for photoelectrons.
Understanding the factors that determine the maximum kinetic energy of photoelectrons is essential in various fields, including physics, chemistry, and engineering. By controlling the frequency of the incident radiation and the material of the surface, scientists can manipulate the maximum kinetic energy of photoelectrons for various applications, such as solar cells and X-ray detectors.
