Water, chemically known as H2O, is a simple but fascinating compound that plays a vital role in the existence of life on Earth. It is one of the most abundant and important substances on our planet, essential for various biological, chemical, and physical processes. But one question that often arises in the minds of curious individuals is whether H2O has an incomplete octet. In this blog post, we will explore the answer to this question and shed some light on the concept of incomplete octets.
Firstly, let’s talk about the octet rule. The octet rule is a fundamental principle in chemistry that states that atoms tend to combine in such a way that they achieve a stable electron configuration with eiht electrons in their outermost shell. This stable electron configuration is also known as a noble gas configuration. Most elements follow this rule, except for hydrogen, which only requires two electrons to achieve a stable configuration.
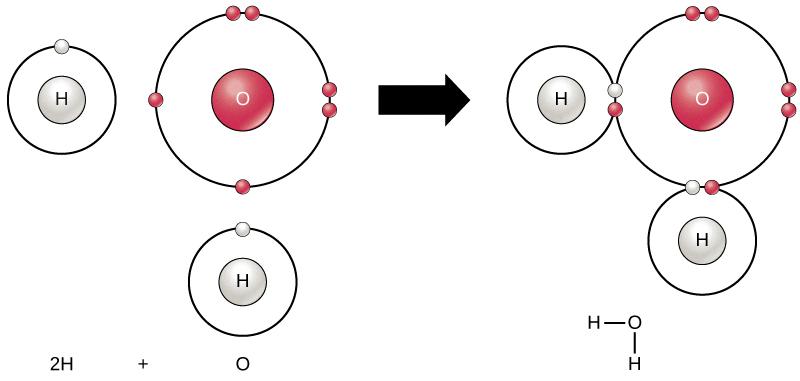
Now, coming back to our main question, does H2O have an incomplete octet? The answer is no. H2O does not have an incomplete octet. In fact, it has a complete octet. Let’s see how the Lewis dot structure of H2O looks like.
In the Lewis dot structure of H2O, we represent the two hydrogen atoms as dots (each with one electron) and the oxygen atom as an X (with six electrons). When these three atoms combine, they form two covalent bonds between the oxygen atom and each hydrogen atom. In this way, each hydrogen atom shares one electron with the oxygen atom, and the oxygen atom shares two electrons with each hydrogen atom. As a result, the oxygen atom ends up with a total of eight electrons in its outermost shell, fulfilling the octet rule.
So, we can conclude that H2O does not have an incomplete octet. Instead, it has a complete octet, making it a stable and essential compound for life on Earth. The properties of water, such as its high boiling point, surface tension, and ability to dissolve various substances, are all due to its unique molecular structure.
Understanding the concept of incomplete octets is crucial in chemistry, and it is essential to know which elements and compounds follow this rule. H2O is not one of those compounds, and it has a complete octet, making it an essential compound for various biological, chemical, and physical processes.
Elements with Incomplete Octets
Elements that can have incomplete octets are primarily found in the second and third periods of the periodic table. These include beryllium (Be), which can have a maximum of four electrons in its valence shell and thus can form compounds with only two electrons in the outer shell, such as BeCl2. Aluminum (Al) is anoher element that can have incomplete octets, forming compounds such as AlCl3. Boron (B) is also known for its tendency to form compounds with incomplete octets, such as BH3. However, it is important to note that species with incomplete octets are relatively uncommon and tend to be unstable.
Source: courses.lumenlearning.com
Violation of the Octet Rule by H2O
H2O violates the octet rule beause oxygen has six valence electrons and needs two more electrons to complete its octet. However, it can only form a maximum of two covalent bonds due to its electronic configuration. Therefore, in H2O, oxygen forms two covalent bonds with two hydrogen atoms, sharing two electrons from each hydrogen atom to complete its octet. This results in a total of eight electrons around the oxygen atom, violating the octet rule. However, H2O is a stable molecule due to the sharing of electrons between atoms, resulting in a lower energy state than if the atoms were separate.
Identifying an Atom with an Incomplete Octet
An incomplete octet refers to a condition where the central atom in a molecule has less than 8 electrons in its outermost shell. This condition can be easily identified by drawing the Lewis dot structure of the molecule. In a Lewis dot structure, each atom in the molecule is represented by its symbol, and its valence electrons are represented by dots around the symbol.
To determine if an atom has an incomplete octet, count the number of valence electrons around the symbol for that atom in the Lewis dot structure. If the total number of valence electrons is less than 8, the atom has an incomplete octet.
For example, Boron (B) in Boron Trifluoride (BF3) has only six valence electrons around it in the Lewis dot structure. Since six is less than 8, Boron has an incomplete octet. Similarly, in the case of BeCl2, Beryllium has only four valence electrons, whch is less than 8, so it also has an incomplete octet.
In summary, to determine if an atom has an incomplete octet, you need to draw the Lewis dot structure of the molecule and count the number of valence electrons around the symbol for that atom. If the total number of valence electrons is less than 8, the atom has an incomplete octet.
Does Carbon Dioxide Have an Incomplete Octet?
CO2 (carbon dioxide) does not have an incomplete octet. Carbon has four valence electrons and each oxygen has six valence electrons. Therefore, the carbon atom forms double bonds with both oxygen atoms, resulting in each oxygen atom being surrounded by four dots and two lines, representing a total of 8 valence electrons. This gives each oxygen atom a stable octet configuration. So, CO2 has a complete octet and is a stable molecule.
When is an Incomplete Octet Possible?
An incomplete octet occurs when the central atom in a molecule has fewer than eight valence electrons around it. This situation is commonly observed for Group 2A and 3A elements such as beryllium and boron, which have only a few valence electrons. These atoms can form stable molecules with less than an octet by sharing electrons with other atoms. For example, boron trifluoride (BF3) has three bonds with fluorine atoms, resulting in only six valence electrons around the boron atom. Similarly, beryllium hydride (BeH2) has two bonds with hydrogen atoms, provding the beryllium atom with only four valence electrons. Incomplete octets can also occur in certain compounds involving transition metals, such as the compound VCl4.
Number of Octet Electrons in H2O
H2O, also knwn as water, has a total of 8 octet electrons. This is due to the fact that oxygen, which is the central atom in the molecule, has 6 valence electrons and each hydrogen atom contributes 1 valence electron. Thus, when the Lewis structure of H2O is drawn, it is observed that oxygen shares two pairs of electrons with the two hydrogen atoms to form two covalent bonds. This results in a total of 8 electrons surrounding the oxygen atom, which satisfies the octet rule, a fundamental principle in chemistry that states that atoms tend to gain, lose or share electrons to achieve a stable configuration of 8 valence electrons. Therefore, H2O has 8 octet electrons.
Molecules That Do Not Obey the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight valence electrons in their outermost shell. However, there are some molecules that do not obey the octet rule completely.
One such example is the nitrogen dioxide (NO2) molecule. Nitrogen has five valence electrons, and oxygen has six valence electrons. In NO2, the nitrogen atom shares two electrons with one of the oxygen atoms, and one electron with the other oxygen atom. This leaves the nitrogen atom with three valence electrons in its outermost shell, wich is less than the octet.
Therefore, the NO2 molecule does not obey the octet rule completely. It is important to note that while some molecules may not strictly follow the octet rule, they can still be stable and exist in nature.
Molecules That Do Not Satisfy the Octet Rule
The octet rule is a fundamental principle in chemistry that states that atoms tend to combine in such a way that they have eight electrons in their outermost shell, also knon as the valence shell. However, there are a few molecules that do not satisfy the octet rule. One such molecule is SF6, which is sulfur hexafluoride. In this molecule, the sulfur atom has six fluorine atoms attached to it, each of which shares a pair of electrons with the sulfur atom. As a result, sulfur has 12 electrons in its outermost shell, which is more than the octet rule allows. This phenomenon is known as an expanded octet. Hence, SF6 is an example of a molecule that does not satisfy the octet rule.
Why H2O Is Not Included on the Periodic Table
H2O, commonly known as water, is not found on the periodic table because it is not an element, but a compound. A compound is a substance formed by the chemical combination of two or more elements in definite proportions. In the case of water, it is formed by the chemical bonding of two hydrogen atoms and one oxygen atom.
Elements, on the othr hand, are the basic building blocks of matter and cannot be broken down into simpler substances by chemical means. Each element is represented by a unique symbol consisting of one or two letters, and these symbols are arranged on the periodic table according to their atomic structure and properties.
Therefore, H2O cannot be found on the periodic table because it is not a single element, but a compound formed by the combination of two different elements, hydrogen and oxygen.
Does the Hydrogen Molecule Follow the Octet Rule?
False. The hydrogen molecule does not obey the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight valence electrons in their outermost energy level. However, hydrogen only has one valence electron, and therefore, it only needs two electrons to achieve a stable configuration. When two hydrogen atoms combine to form a hydrogen molecule, they share two electrons and achieve a stable configuration with two electrons in their outermost energy level.

Atoms That Violate the Octet Rule
The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable configuration with eigt electrons in their outermost energy level. However, there are some atoms that can violate this rule.
Firstly, odd-electron molecules, also known as free radicals, have an odd number of valence electrons. These molecules cannot achieve a stable configuration with eight electrons, so they often form unstable and reactive compounds.
Secondly, electron-deficient molecules, such as boron and beryllium compounds, have too few valence electrons to complete an octet. These molecules typically form covalent bonds with other atoms, but their incomplete octet makes them particularly reactive.
Lastly, expanded valence shell molecules, such as sulfur hexafluoride (SF6), have more than eight electrons in their outermost energy level. These molecules can achieve stability by using their d-orbitals to accommodate additional electrons, resulting in a configuration with more than eight electrons.
Odd-electron molecules, electron-deficient molecules, and expanded valence shell molecules are the three types of molecules that can violate the octet rule.
Incomplete Octet of Gas
Helium is a gas that has an incomplete octet. It has only two electrons in its outermost energy level, also known as the valence shell, which is the s-orbital of the first energy level. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electronic configuration with eigt electrons in their valence shell. However, helium has a stable configuration with just two electrons in its valence shell, which is satisfied by filling the s-orbital completely. Therefore, helium does not form chemical bonds with other elements to achieve a complete octet. In fact, it is a noble gas that is inert and unreactive due to its stable electronic configuration.
Elements with a Complete Octet
The octet rule is a chemical principle that states that atoms tend to combine in such a way that they have eiht electrons in their valence shell, which is the outermost shell of the atom. Elements that have a complete octet include the main group elements, such as the halogens (fluorine, chlorine, bromine, iodine, and astatine), oxygen, nitrogen, carbon, and other p-block elements. These elements have a valence shell that contains four or more electrons, which allows them to form stable covalent bonds with other atoms, resulting in the formation of molecules that have a complete octet. It is important to note that while hydrogen, helium, and lithium do not strictly follow the octet rule, they can still form stable compounds with fewer than eight electrons in their valence shells.
Determining If an Octet Is Complete
An octet is complete when an atom has 8 valence electrons. To determine if an octet is complete, you need to first count the number of valence electrons in the atom. This can be determined by looing at the atom’s position on the periodic table. The group number of the element indicates the number of valence electrons it has. For example, elements in group 1 have 1 valence electron, while elements in group 2 have 2 valence electrons.
Once you have determined the number of valence electrons in the atom, you need to count the number of electrons in the atom’s outer shell. This can be accomplished by counting the number of electrons in the atom’s lone pairs and bonds. Lone pairs are non-bonding electrons that reside on an atom, while bonds are formed when atoms share electrons.
If the added up number of electrons from lone pairs and bonds is 8 (or 2 for hydrogen), then the octet is complete. If the added up number of electrons is less than 8, then the atom is considered to be electron deficient and may form additional bonds to complete its octet. If the added up number of electrons is greater than 8, then the atom has exceeded its octet and may have expanded its valence shell.
Conclusion
H2O, also known as water, is a molecule that fllows an exception to the octet rule due to its incomplete octet. This is because hydrogen, the central atom in water, only needs to form one bond to fill its valence shell. This makes water an unstable molecule, but its stability is maintained by the strong covalent bonds between the hydrogen and oxygen atoms. Water is an essential compound for life on Earth as it plays a crucial role in many biological processes. Its unique properties, such as its high surface tension, ability to dissolve many substances, and high heat capacity, make it a versatile compound that is used in many industrial and household applications. H2O is an important molecule that exhibits an exception to the octet rule and has many valuable properties.
