Have you ever wondered what it means for a molecule to be protonated or deprotonated? It’s an important concept in chemistry that can determine the reactivity of a compound, and understanding it can help us better understand how different molecules interact with each other.
What is protonation and deprotonation? Protonation is the process of adding a proton (or hydrogen cation, H+) to an atom, molecule, or ion. This creates a conjugate acid, which is the protonated form of the molecule. The opposite process is called deprotonation, which is when a proton is removed from a Brønsted–Lowry acid. This creates the base form of the molecule.
The difference between protonation and deprotonation depends on the pH level – this is determined by the pKa of each functional group on an amino acid. At a pH below the pKa for each functional group on an amino acid, that group will be protonated; at a pH above it will be deprotonated; and if the pH equals the pKa then that functional group will be 50% protonated and 50% deprotonated.
It’s important to understand these concepts because they can determine how reactive different molecules are with each other depending on their state – wheher they are protonated or deprotonated. Knowing this can help us better understand chemical reactions and how different compounds interact with each other in various environments.
Identifying Protonation and Deprotonation
The acidity or basicity of a molecule is determined by its pKa value. Each functional group on an amino acid has a different pKa associated with it. If the pH of the solution is blow the pKa for a particular functional group, then that functional group is protonated, meaning it has gained a hydrogen atom. If the pH of the solution is above the pKa for that functional group, then that functional group is deprotonated, meaning it has lost a hydrogen atom. At a pH equal to the pKa, then the functional group is equally likely to be either protonated or deprotonated.
What Is Deprotonation?
Deprotonation is a chemical reaction in which an acid loses a proton (H+) from its molecular structure. This reaction can occur when an acid is exposed to a base, such as sodium hydroxide (NaOH). The resulting product of deprotonation is an ionic compound known as the conjugate base, which is a negatively charged species. By removing the proton from the original molecule, the acid has been “deprotonated” and converted into its conjugate base. Deprotionation reactions are important in many areas of chemistry, including organic synthesis, biochemistry, and drug design.
The Meaning of Protonated and Deprotonated
Protonation is the process of adding a proton (or hydrogen cation, H+) to an atom, molecule, or ion, forming a conjugate acid. This is the opposite of deprotonation, which is the removal of a proton from a Brønsted–Lowry acid. In other words, protonated molecules (or ions) have an extra hydrogen atom attached to them (forming H+), while deprotonated molecules (or ions) have lost a hydrogen atom and now contain only one electron.
The Protonation and Deprotonation of Acids
Acids are protonated when they are in their acidic form. This means that they have an extra hydrogen atom (H+) attached to them. When acids are deprotonated, they lose the H+ and become their basic form. This means that the acid can act as both an acid and a base depending on its protonation state.
Does Protonation Mean an Uncharged Molecule?
No, protonated does not necessarily mean uncharged. The protonated form of an acid is uncharged (neutral), but the protonated form of a base will be charged. In other words, when a compound is protonated, it gains or loses a hydrogen ion, depending on whether the compound is an acid or a base. If it’s an acid, the hydrogen ion will be added to the molecule and the resulting compound will be neutral. If it’s a base, the hydrogen ion will be removed from the molecule and the resulting compound will be charged.
Source: chemistry.stackexchange.com
The Charge of Protonation
The protonated form of a molecule is positively charged. This occurs when one or more hydrogen atoms (protons) become attached to the molecule, giving it an overall positive charge. The protonation of molecules can occur in a variety of ways, including through the use of acids or bases, or due to the presence of an electrostatic field.
The Impact of Protonation on pH Levels
Protonation does not directly increase pH. Protonation is the process of adding a proton (H+) to an atom or molecule, resulting in the formation of an ionic bond. This process will make the solution more acidic, since there are more positively charged ions present. However, it is important to note that while protonation can decrease pH, it cannot increase it; in order to increase the pH of a solution you must add a base (such as hydroxide ions) which will consume free protons and thus raise the pH value.
The Effects of Deprotonation on pH
Deprotonation of carboxylic acid groups occurs at a pH of around 7, which is the physiological pH of most living organisms. This means that when the pH of the environment is close to 7, these amino acid side chains will be mainly in their deprotonated form.
Is Deprotonation Equivalent to Acidity?
No, deprotonation does not mean acidic. Deprotonation is the process of removing a proton from an acid, thereby converting it into a different species. It can be done by a base, which donates electrons to the acid and removes its most acidic proton. The result is an anion (negatively charged ion) and a conjugate base (the substance that was used to deprotonate the acid). An example of this woud be when hydrochloric acid (HCl) is deprotonated by sodium hydroxide (NaOH), forming the anion chloride (Cl-) and the conjugate base sodium ion (Na+). The resulting anion is not necessarily acidic, as it may be neutral or even basic depending on its structure.
Identifying Protonated Atoms
To identify which atom is protonated, you must first scan the molecule for all non-halogen atoms with lone pairs (usually N and O). These are the candidate atoms that can be protonated. Then, imagine protonating each of these candidate atoms and draw its conjugate acid. By comparing the conjugate acids, you can identify which one is the weakest. The protonated atom in the weakest conjugate acid is the most basic atom in the original molecule and therefore the one that has been protonated.
Acidity and Basicity of Protonated Compounds
Protonated compounds are more acidic than their unprotonated forms. This is because when a compound is protonated, it gains an additional hydrogen atom, which increases the overall acidity of the compound. This increase in acidity is due to the increased concentration of protons in solution and the increased availability of protons for reaction with other molecules. Thus, protonation makes a compound more acidic than it was before.
The Protonation/Deprotonation of Acids at Low pH
At low pH, acids are protonated. This means that the hydrogen ion (H+) from the acidic environment attaches itself to the acid molecule, forming a positively-charged species. The ability for an acid to donate a proton is what gives it its acidic character. In contrast, at higher pH levels, the hydrogen ions are removed from the acid molecules, leaving them in their deprotonated form. This results in a negatively charged species which no longer has the ability to donate protons and is therefore neutralized.
What is Deprotonation in Chemistry?
In chemistry, deprotonation is the process of removing a proton from a molecule. This can be achieved by reacting the molecule with a base, such as sodium hydroxide (NaOH). Deprotonation usually refers to the chemical reaction between an acid and a base, which results in the formation of a salt and water. In this case, the proton is transferred from the acid to the base, causing the acid to become deprotonated. Deprotonation can also refer to other processes in which protons are removed from molecules, such as electrochemical reactions or chemical oxidation.
The Ionization State of Deprotonated and Protonated Molecules
Ionized compounds can be either deprotonated or protonated, depending on the pH of their environment. In solutions with a pH lower than the pKa of the compound, the compound will be protonated and neutral in charge. Conversely, if the pH is higher than the pKa of the compound, then it will be deprotonated and negatively charged (ionized). Acids are neutral when protonated and negatively charged (ionized) when deprotonated. Bases, on the other hand, are neutral when deprotonated and positively charged (ionized) when protonated.

The Protonation of Acids
Yes, acids can be protonated. Protonation is a type of reaction in which a proton (H+) is added to an acid, resulting in the formation of a new compound. When an acid is protonated, the hydrogen atom of the acid binds with a counterion such as water or hydroxide and forms an ionic bond. This results in a new species known as the conjugate base.
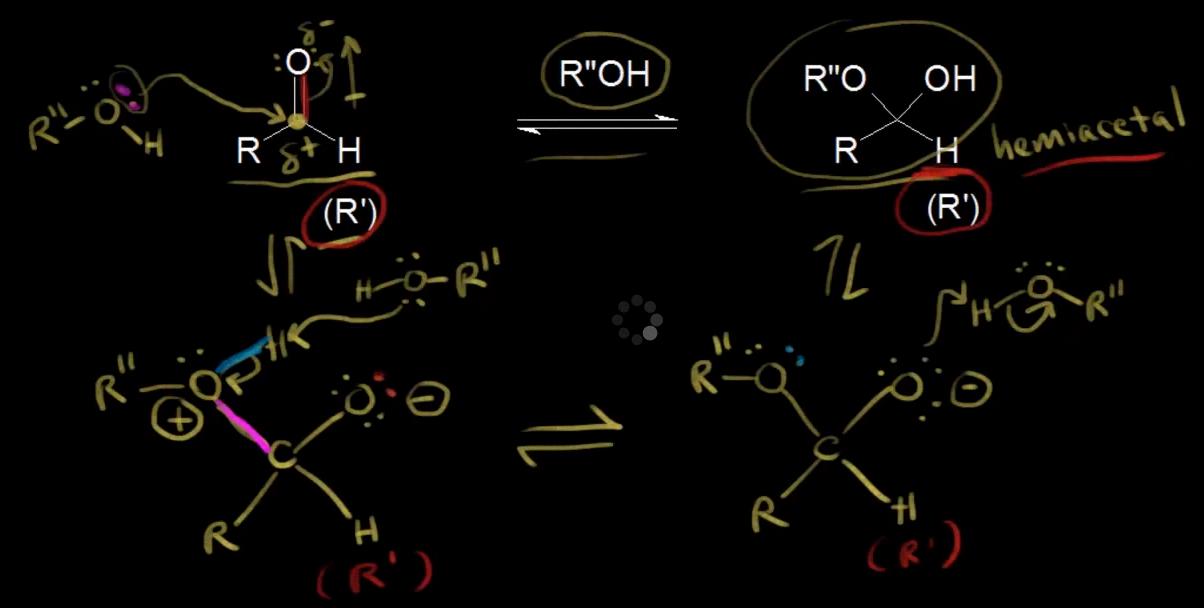
In organic chemistry, acids can be protonated by various mechanisms, including Fischer esterification, nucleophilic substitution reactions, and addition-elimination reactions. In these reactions, the hydrogen atom from the acid binds with a nucleophile such as an alcohol or amine and forms an intermediate species known as an oxonium or azanium ion. This intermediate then undergoes a rearrangement reaction to form the desired product.
Protonation plays an important role in many biochemical processes such as enzyme catalysis and cell signaling pathways. Protonation of specific amino acids in proteins can result in changes to protein structure and function wile also allowing proteins to bind to other molecules. Protonation also plays an important role in regulating pH levels within cells and the body’s extracellular environment by controlling the concentration of protons available for binding with other molecules.
Conclusion
In conclusion, the protonation or deprotonation of an amino acid is dependent on the pH of its environment. At a pH below the pKa for each functional group on the amino acid, the functional group is protonated. At a pH above the pKa for the functional group it is deprotonated. If the pH equals the pKa, then the functional group is 50% protonated and 50% deprotonated. Thus, depending on its environment, an amino acid can exist in either its protonated or deprotonated form.
