CH3COOH, also known as acetic acid, is one of the most important organic acids in chemistry. It is a weak acid that consists of a hydroxyl group (OH-) covalently bonded to a methyl group (CH3). The molecule is composed of two types of atoms: carbon and oxygen. The hydrogen atom in the CH3 group can be replaced with other atoms or molecules to form a variety of derivatives.
When CH3COOH dissolves in water, it forms the CH3COO- anion and H+ cations. This process is known as ionization and occurs when the hydrogen atom in the CH3 group takes away an electron from the oxygen atom in the OCO group, forming an overall negative charge on the molecule. The H+ ions then react with water molecules to form hydronium ions (H3O+).
The CH3COO- anion and H+ cation are held tgether by ionic bonds, which are much stronger than covalent bonds. Ionic bonds occur when two oppositely charged ions attract each other due to their electrostatic force of attraction. In contrast, covalent bonds occur when two atoms share electrons with each other due to their mutual electronegativity.
CH3COOH is an important compound used in many industrial processes such as fermentation and food preservation. It also serves as a precursor for many compounds such as esters, amines, and alcohols. As you can see, understanding how this molecule behaves can help us improve our lives!
Ionic or Covalent Nature of CH3COONA
CH3COONa, or Sodium acetate, is an ionic compound. This means that it is composed of two oppositely-charged ions: the acetate anion (CH3COO-) and the sodium cation (Na+). The positive sodium cation is attracted to the negative acetate anion, forming a strong ionic bond between them. Although the carbon-oxygen bonds within the acetate anion are covalent in nature, the overall compound itself is considered to be ionic due to its overall charge.
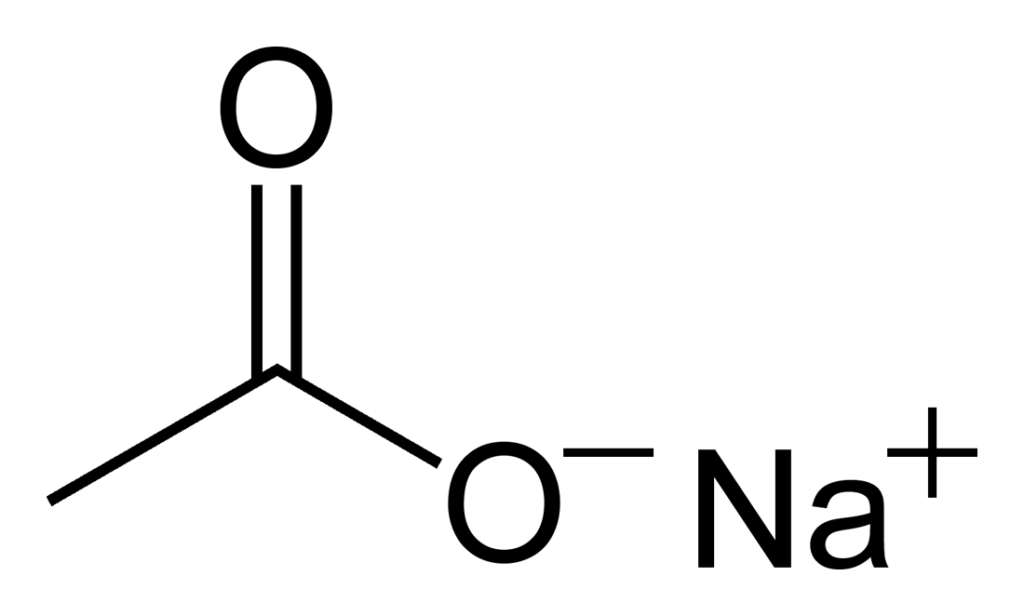
What Is the Chemical Composition of CH3COONa?
CH3COONa, or Sodium Acetate, is an inorganic compound composed of a single sodium ion (Na+) and one acetate anion (CH3COO-). It is a white, hygroscopic crystalline solid which is very soluble in water. Sodium acetate has a wide range of uses including as a food additive, in industry, for concrete manufacture, heating pads and buffer solutions. It also has medical applications, acting as an electrolyte replenisher when given intravenously.
Is CH3COONa an Ion?
No, CH3COONa is not an ion. It is an ionic compound, which consists of a cation (sodium ion, Na+) and an anion (acetate ion, CH3COO-). Ionic compounds form when cations and anions attract each other in a chemical reaction to form a neutral compound. The individual ions that make up the compound are held together by the electrostatic forces between them.
The Covalent Nature of Acetic Acid
Acetic acid is covalent because its carbon atom has four electrons in its outer shell. This means it can form a covalent bond with other molecules by sharing electrons with them. The shared electrons create a strong connection between the molecules, forming a covalent bond. Covalent bonds are generally more stable than ionic bonds and can hold molecules together more securely.

Conclusion
Acetic acid, or CH3COOH, is a weak acid made up of a hydrogen cation (H+) and an acetate anion (CH3COO-). Its molecules are held together by covalent bonds and it is one of the simplest carboxylic acids. Acetic acid has a sour taste, and it is slightly soluble in water. It can be used as a food preservative, and it can also be used in the production of many other products, such as plastics, pharmaceuticals and dyes.
