Have you ever wondered why certain materials can absorb and release energy more efficiently than others? The answer lies in the concept of specific heat, which is a measure of how much energy is required to increase the temperature of a material by one degree. It is also important to note that specific heat can be either positive or negative, depending on the material in question.
Specific heat is defined as the amount of energy (in Joules) needed to raise the temperature of one gram of a substance by one degree Celsius. It is a measure of how much heat energy is needed to increase or decrease the temperature of an object by a certain amount. The higher the specific heat value, the more energy it takes to change its temperature and vice versa.
The most common example used to illustrate this concept is water. Water has a very high specific heat value (4.186 J/g°C), meaning that it takes a large amount of energy to raise its temperature by just one degree Celsius. This explains why it takes a lot longer for water to boil compared to othr liquids with lower specific heats such as alcohol (2.4 J/g°C).
It is important to note that specific heats can also be negative in some situations. Negative specific heats occur when adding energy actually causes temperatures to drop instead of rise, due to an entropy effect caused by increasing disorder in certain systems. This effect has been observed in several condensed matter systems such as molecular gases and superconductors, but has also been found in some biological systems such as cells and organisms.
In conclusion, understanding how specific heats work can give us insights into why certain substances behave differently than others when exposed to different temperatures or amounts of energy. It also helps us understand why negative specific heats occur and what kind of effects they have on various systems ranging from molecules all the way up to living organisms!
Can Specific Heat Have a Positive or Negative Value?
Answer: Specific heat can be both positive and negative. Positive specific heat means that the material absorbs energy (heat) when its temperature increases, while negative specific heat means that the material releases energy (heat) when its temperature increases. In oter words, materials with positive specific heat require an input of energy to increase their temperature, whereas materials with negative specific heat release energy when their temperature increases. In general, solids tend to have positive specific heat and gases tend to have negative specific heat; however, there are exceptions to this rule. For example, saturated vapour has a negative specific heat due to the fact that it releases energy when it condenses into liquid form.
Can Negative Specific Heat of a Metal Exist?
No, the specific heat of a metal cannot be negative. Specific heat is the amount of energy required to raise the temperature of a unit mass of a substance by one degree Celsius. It is always positive because it takes energy to raise the temperature of any substance.
Negative Specific Heat: Exploring the Phenomenon
The specific heat of a material is the amount of energy required to raise its temperature by one degree. When energy is removed from a material, its temperature increases, so the specific heat must be negative. This can be explained using the Maxwell-Boltzmann distribution, which relates velocity to temperature. As energy is removed from a material, its atoms will descend into lower orbits and their velocity will increase, thus raising their temperature. Therefore, the specific heat must be negative in order to account for this increased temperature when energy is taken away.
Negative Heat Capacity: Is It Possible?
Yes, it is possible to have a negative heat capacity. This occurs when the average kinetic energy of a system increases as energy is removed from the system. This can happen when the system radiates energy into space, or when energy from an outside source is removed from the system and the internal energy of the system increases. For example, if a certain amount of heat is removed from a gas, its temperature could decrease significantly due to an increase in its internal energy; this would result in a negative heat capacity.
Does Specific Heat Have a Positive Value?
Yes, specific heat is always positive. Specific heat is the amount of energy required to raise the temperature of a unit mass of a substance by one degree Celsius. Since energy is being added to the system, not taken away, the specific heat must be positive.
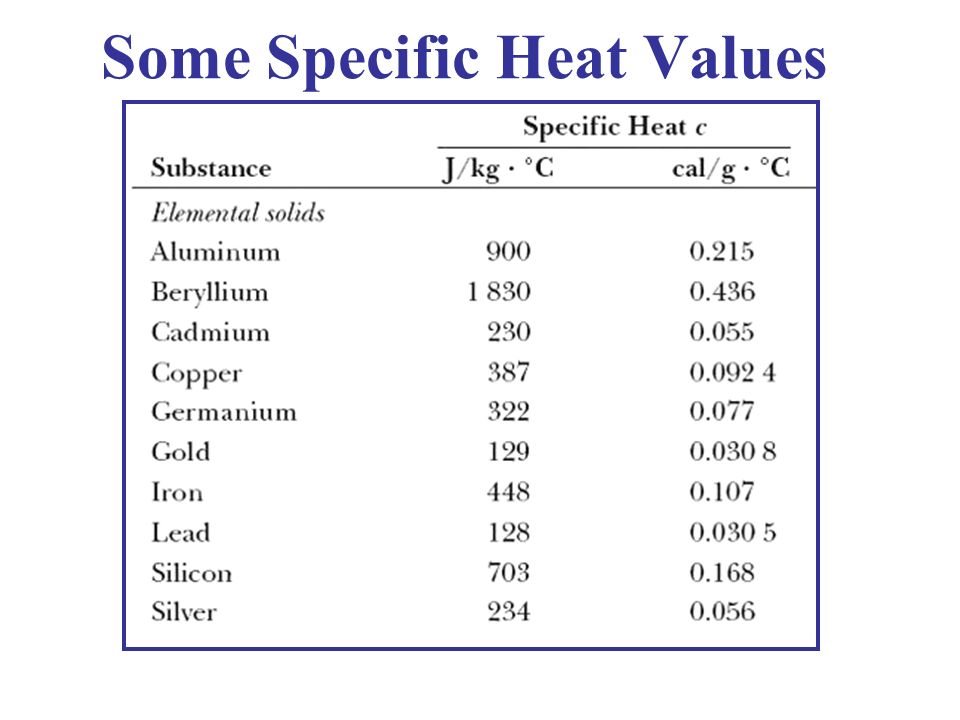
Can Specific Heat Have a Zero, Infinity, or Negative Value?
Yes, specific heat can have a value of zero, infinity or even negative. Specific heat is defined as the amount of energy that neds to be added to a unit mass of a substance in order to increase its temperature by one degree. It is usually measured in units of joules per kilogram kelvin (J/kg·K).
At temperatures close to absolute zero (0 K), the specific heat of many materials tends to approach zero because the thermal energy required for increasing temperature becomes very small. Likewise, at temperatures approaching infinity, the specific heat tends to approach infinity as it takes an infinite amount of energy to increase the temperature by one degree.
In addition, some substances such as hydrogen gas have a negative specific heat which means that when energy is added to them their temperature decreases rather than increases. This is due to their unique atomic structure and intermolecular forces which allow them transfer energy among molecules more efficiently than other substances.
Negative Specific Heat Capacity
Yes, the Q in specific heat can be negative. This typically occurs when heat is beng transferred from a system to the environment, or when heat is being absorbed from a solution. In both cases, the heat transfer will result in a negative Q for the system or solution, as more energy is being removed from it than added to it. For example, when ice melts and changes from a solid to a liquid state, energy is released into the environment and Q for the system will be negative. Likewise, when heat is absorbed from a solution containing an exothermic reaction, q for the solution will also have a negative value.
Can Negative Specific Heat Occur in Gases?
Yes, specific heat can be negative for a gas. This happens when the temperature of the gas is decreasing and the energy released from the gas is greater than the energy absorbed by it. In this case, the specific heat capacity of the gas is negative, meaning that it takes more energy to raise its temperature by one degree than it releases when its temperature is decreased by one degree. This phenomenon is known as a “negative Joule-Thompson effect”.
The Meaning of a Negative Heat Value
A negative heat value indicates that the reactants have a higher enthalpy than the products, meaning that energy is released during the reaction. This energy can be in the form of heat or light, making it an exothermic reaction. In an exothermic reaction, energy is released from the system and can be used to do work.

Can Specific Heat Have a Value of Zero?
Yes, the specific heat capacity of a substance can be zero. Specific heat capacity is defined as the amount of heat energy required to raise the temperature of unit mass of the substance through 1°C or 1 K. A substance with a specific heat capacity of zero wuld require no energy to raise its temperature, meaning it would have no thermal inertia and thus be unable to store or transfer any heat energy. This phenomenon is usually observed in gases, which tend to respond quickly to changes in temperature. However, other substances such as certain liquids and solids can also have a specific heat capacity of zero or close to zero.
Can Negative Joules Represent Heat?
Yes, heat can be negative joules. Heat is a form of energy, and like any other form of energy, it can be either positive or negative. A positive value for heat (+q) means that heat is entering the object, while a negative value for heat (-q) means that heat is leaving the object. For example, if the temperature of an object decreases by 1 degree Celsius and it releases 3 J of energy, then the amount of heat lost is -3 J. In other words, the object has lost 3 J of energy due to a decrease in temperature.
Conclusion
Conclusion: Specific heat is a measure of the energy required to raise or lower the temperature of an object. It is defined as the amount of heat required to raise the temperature of one kilogram of a substance by one degree Celsius. In some cases, such as when dealing with saturated vapour, specific heat can be negative, meaning that as the temperature increases, heat is released from the system. Overall, understanding specific heat can help us better understand how energy interacts with matter on a molecular level.
