Welcome to the blog post all aout AuCl3, or gold trichloride! Gold trichloride is an inorganic compound composed of gold and chlorine with the chemical formula AuCl3. This compound is most often prepared by passing chlorine gas over gold powder heated to 180 degrees Celsius (356 degrees Fahrenheit).
Gold trichloride is highly hygroscopic and very soluble in both water and ethanol. It begins to decompose at temperatures higher than 160 degrees Celsius or when exposed to light. Additionally, it forms a variety of complexes with many ligands.
The applications for gold trichloride are quite varied. This compound can be used as a reagent in organic synthesis, as a catalyst for certain reactions, and as a precursor for preparing other compounds containing gold such as gold(III) bromide, chloride, and iodide. It can also be used in the fabrication of semiconductor devices due to its ability to react with silicon dioxide and form silicon tetrachlorogoldate(III), which has interesting electrical properties.
Gold trichloride can also be used in varios laboratory procedures such as qualitative analysis tests and colorimetric tests, as well as in electroplating processes. It’s even been found to be useful in the synthesis of pharmaceuticals such as anti-inflammatory drugs!
Clearly, AuCl3 is an incredibly versatile compound with a variety of applications across industry sectors and research fields. If you have any questions about this valuable chemical compounds feel free to reach out and we’ll be more than happy to prvide you with additional information!
Formation of AuCl3
Gold(III) chloride, also kown as AuCl3, is formed by passing chlorine gas over gold powder at a temperature of 180°C (356°F). This reaction produces a mixture of gold metal and elemental chlorine gas. The chlorine gas then combines with the gold metal to produce AuCl3 molecules. This reaction is exothermic, meaning it releases energy in the form of heat. The reaction can be written as: 2Au + 3Cl2 ? 2AuCl3.
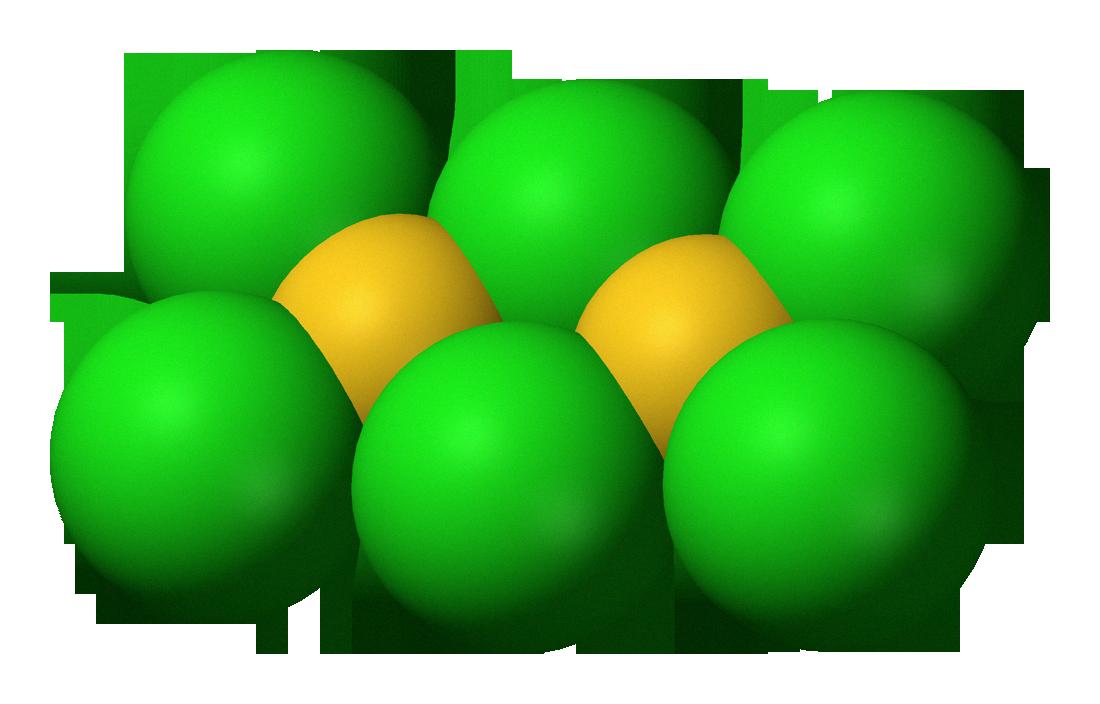
Source: commons.wikimedia.org
Solubility of AuCl3
Gold(III) chloride, or AuCl3, is highly soluble in water and ethanol. It forms complexes with many ligands and breaks down abve 160 °C or in light. Therefore, it can be said that AuCl3 is soluble.
Uses of AuCl3
AuCl3, also known as chlorauric acid or gold trichloride, is a chemical compound with a variety of uses. It is most commonly used in photography as a toner to give black and white photographs a more vivid look. It can also be used in the production of ceramic glazes, enameling glass, creating Ruby glass and gold plating objects. Further applications include the production of fine gold powder for industrial use.
Charge of Au in AuCl3
The charge of Au in AuCl3 is +3. This is because all halogens have an oxidation number of -1 in compounds made up of two atoms, and sice Cl has an oxidation number of -1, Au must have an oxidation number of +3 to balance the charge.
Preparing Gold Nanoparticles
Gold nanoparticles can be prepared throgh a variety of methods, including chemical reduction, laser ablation, and electrochemical synthesis. The most common method involves the use of citric acid as a reducing and stabilizing agent. This method was first developed in 1951 by treating hydrogen tetrachloroaurate (HAuCl4) with citric acid in boiling water. The citric acid acts as a reducing agent to reduce the Au+4 ions to Au0 particles, which are then stabilized by the citrate molecules that form an outer shell around the particles. Other reducing agents such as sodium borohydride (NaBH4) and hydroxylamine hydrochloride (NH2OH·HCl) have also been successfully employed in preparing gold nanoparticles. In addition, it is possible to produce gold nanoparticles with different sizes and shapes through varying the concentrations of the reagents and reaction conditions such as temperature, pH, stirring rate, reaction time, etc.
Is AuCl3 a Salt?
Yes, AuCl3, or Aurous Gold Chloride, is a salt. It is a white, crystalline solid that is produced by heating Gold Trichloride (AuCl3) to 185°C in air for twelve hours. The resulting compound contains ions of gold and chlorine in a 1:3 ratio. It has low solubility in water and reacts with acids to form other gold compounds such as chlorauric acid (HAuCl4). The compound has been studied for its possible use in electroplating and other industrial applications.
The Composition of Aqua Regia
Aqua regia is a mixture of three parts concentrated hydrochloric acid (HCl) and one part concentrated nitric acid (HNO3). It is an acidic, corrosive, and oxidative solution that can dissolve gold and platinum. The hydrochloric acid provides the acidic environment needed to break down gold and other noble metals, while the nitric acid helps to oxidize them into soluble compounds. When combined in the correct proportions, aqua regia is able to dissolve many diffrent precious metals that would otherwise be insoluble in either of the acids alone.
The Meaning of Au in Gold
Au is the chemical symbol for gold, derived from the Latin word aurum, meaning “shining dawn.” Gold is a precious metal known for its attractive yellow luster and resistance to corrosion. It has been used in jewelry and electronics since ancient times. Gold atoms are held together with strong chemical bonds, making it one of the densest and heaviest elements on Earth.
Conclusion
In conclusion, Gold(III) Chloride, also known as AuCl3, is a hygroscopic and highly soluble compound. It can be prepared by passing chlorine gas over gold powder at high temperatures and decomposes when exposed to light or high temperature. It forms a variety of complexes with many ligands, making it a versatile compound. Its properties make it a valuable tool in various industries and research initiatives.
