Tyrosine is an essential amino acid that is important for various biological processes. It is classified as an aromatic amino acid and is derived from phenylalanine by hydroxylation in the para position. This means that a hydroxyl (-OH) group is added to the benzene ring of phenylalanine to form tyrosine. This addition makes tyrosine different from phenylalanine and gives it unique properties.
One of the properties of tyrosine is solubility in water. Unlike phenylalanine, tyrosine is soluble in water. This is because the hydroxyl group in tyrosine makes it more polar compared to phenylalanine. Polar molecules are those that have a positive and negative charge distribution within the molecule, making them interact with water molecules. Since water is a polar solvent, polar molecules dissolve in it easily. This means that tyrosine can interact with water molecules and dissolve in water.
However, the polarity of tyrosine is not solely based on the presence of the hydroxyl group. In general, the polarity of amino acids is determined by their side chains or R groups. If the R group is polar, then the amino acid is polar. If the R group is nonpolar, then the amino acid is nonpolar. In the case of tyrosine, its R group is a benzene ring with an attached hydroxyl group. This R group is considered hydrophobic or nonpolar. This means that tyrosine is not completely polar, but rather a molecule with polar and nonpolar regions.
It is important to note that the hydroxyl group in tyrosine is rarely found deprotonated at any physiologically relevant pH. This means that the hydroxyl group does not typically have a negative charge, which is a characteristic of polar molecules. Instead, it is usually protonated and has a neutral charge. Therefore, the hydroxyl group in tyrosine does not contribute significantly to its polarity.
Tyrosine is a molecule with both polar and nonpolar regions. Its solubility in water is due to the presence of the hydroxyl group, which makes it more polar compared to phenylalanine. However, its R group is hydrophobic or nonpolar, which makes it less polar compared to other amino acids with polar R groups. The polarity of tyrosine is an important factor in its biological functions and interactions with other molecules in the body.
Is Tyrosine Non-polar Or Polar?
Tyrosine is a polar amino acid due to the presence of hydroxyl (-OH) group on its aromatic ring. This hydroxyl group makes tyrosine soluble in water and othr polar solvents. The polar nature of tyrosine also allows it to form hydrogen bonds with other polar molecules, which is essential for its biological functions. However, it is important to note that tyrosine can have nonpolar regions in its structure, such as the hydrophobic side chains of the amino acid, which can influence its interactions with nonpolar molecules. tyrosine is considered a polar amino acid due to its hydroxyl group and overall solubility in water.
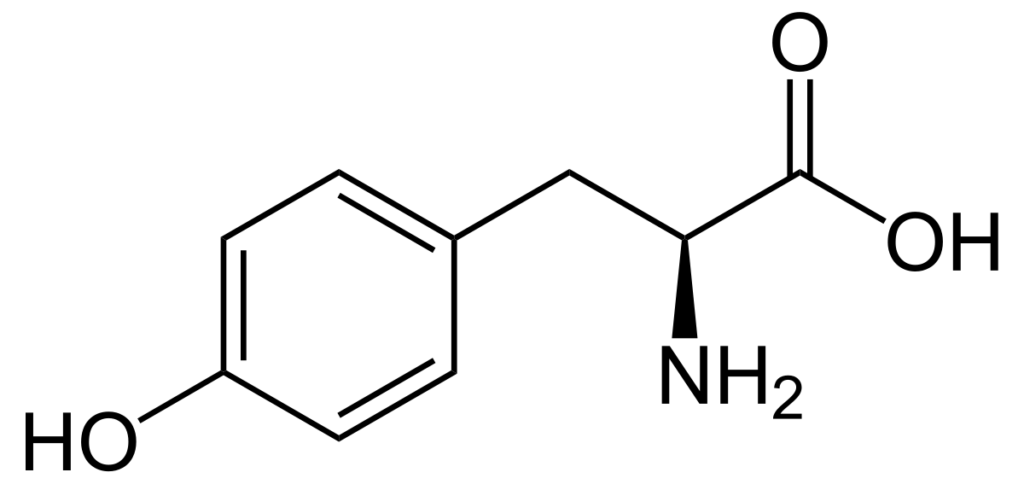
Why Is Tyrosine Polar And Nonpolar?
Tyrosine is an amino acid that has a polar hydroxyl (-OH) group in its side chain, which makes it a polar molecule. However, the rest of its side chain is composed of non-polar hydrocarbon groups, which gives it some non-polar characteristics. the non-polar portion of tyrosine’s side chain dominates its polarity, leading it to be classified as a non-polar amino acid.
Is Tyrosine Polar Or Hydrophobic?
Tyrosine is considered to be a hydrophobic amino acid. However, it is important to note that it contains a polar hydroxyl (-OH) group on its side chain, which makes it more soluble than other hydrophobic amino acids like phenylalanine. This hydroxyl group allows tyrosine to participate in hydrogen bonding with water molecules, making it more polar than other hydrophobic residues. Nonetheless, tyrosine is still primarily classified as a hydrophobic amino acid due to its nonpolar aromatic ring and aliphatic side chain.
Is Tyrosine Polar Or Nonpolar MCAT?
Tyrosine is considered a nonpolar amino acid. It has a benzene ring in its side chain, which is a hydrophobic group that does not interact well with water molecules. Although tyrosine has an -OH group at the end of its side chain, this hydroxyl group is rarely found deprotonated at any physiologically relevant pH, which means it does not act as a polar group. Therefore, tyrosine is classified as a nonpolar amino acid, rather than a polar one.
Conclusion
Tyrosine is an essential amino acid with an aromatic side chain that is derived from phenylalanine by hydroxylation in the para position. Although it possesses an -OH group at the end of its side chain, this hydroxyl group is rarely found deprotonated at any physiologically relevant pH, making it a nonpolar amino acid. Despite beng hydrophobic, Tyrosine is significantly more soluble than phenylalanine, owing to its polar nature. Its role in protein synthesis and metabolism, as well as its potential therapeutic applications, have been studied extensively. Tyrosine is a critical amino acid with numerous biological functions that are of great interest to researchers and health professionals alike.
