Trigonal pyramidal and tetrahedral are two of the most common molecular shapes found in chemistry. These two shapes are important becase they can help us understand the chemical properties of molecules. In this article, we will explore the differences between these two shapes and their significance.
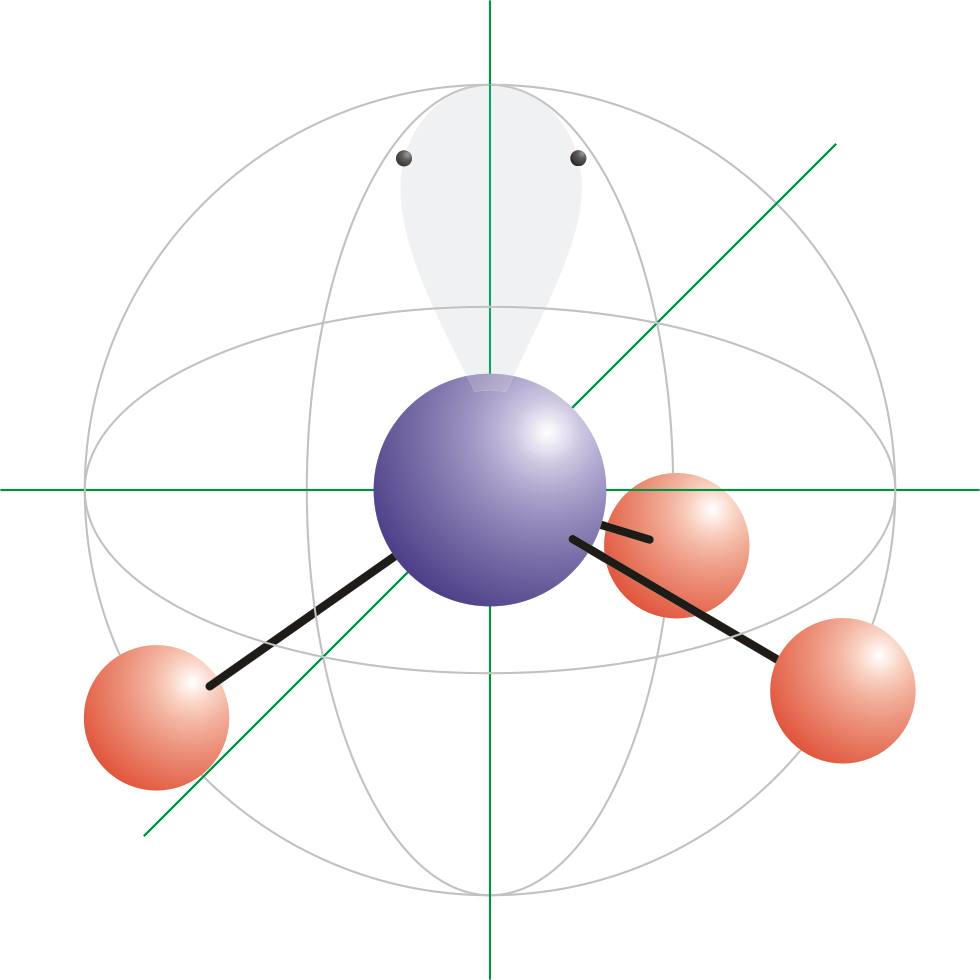
Trigonal pyramidal is a molecular shape that has a central atom surrounded by three atoms and one lone pair. This shape is best illustrated by NH3 (ammonia) molecule. The central nitrogen atom in ammonia has three hydrogen atoms and one lone pair surrounding it. This arrangement gives the molecule a pyramidal shape. The bond angles between the nitrogen and hydrogen atoms are 107 degrees.
On the other hand, tetrahedral is a molecular shape that has a central atom surrounded by four atoms. This shape is best illustrated by CH4 (methane) molecule. The central carbon atom in methane has four hydrogen atoms surrounding it. This arrangement gives the molecule a tetrahedral shape. The bond angles between the carbon and hydrogen atoms are 109.5 degrees.
The main difference between trigonal pyramidal and tetrahedral is the presence of a lone pair of electrons. In trigonal pyramidal, there is one lone pair of electrons at the central atom, whereas in tetrahedral there are no lone pairs. This lone pair in trigonal pyramidal can affect the molecule’s polarity and reactivity. The lone pair can interact with other atoms or molecules, making the molecule more reactive.
Another difference between these two shapes is their symmetry. Tetrahedral is a symmetrical shape because all the bond angles are the same. On the other hand, trigonal pyramidal is not symmetrical because the lone pair of electrons affects the bond angles. The bond angles between the hydrogen atoms are not the same as the angle between the hydrogen atom and the lone pair.
Trigonal pyramidal and tetrahedral are two important molecular shapes that can help us understand the chemical properties of molecules. The presence of a lone pair of electrons in trigonal pyramidal can affect the molecule’s reactivity and polarity, whereas tetrahedral is a symmetrical shape with no lone pairs. Understanding these shapes can help us understand the behavior of molecules in chemical reactions.
Is There A Difference Between Pyramidal And Trigonal Pyramidal?
There is a difference between pyramidal and trigonal pyramidal. Pyramidal refers to the shape of a molecule or ion that has a central atom surrounded by three other atoms or groups of atoms arranged in a triangular shape. This shape can be seen in molecules such as ammonia (NH3) or phosphine (PH3), whee the central atom is bonded to three other atoms in a pyramid-like shape.
On the other hand, trigonal pyramidal refers to a specific type of pyramidal shape where the central atom has one or more lone pairs of electrons. This results in a slightly different shape, with the lone pair(s) occupying a position(s) that is not occupied by a bonding atom. This shape can be seen in molecules such as water (H2O) or nitrogen trifluoride (NF3).
While both pyramidal and trigonal pyramidal refer to a molecular shape with a central atom surrounded by three other atoms or groups of atoms in a triangular arrangement, trigonal pyramidal specifically refers to a shape with one or more lone pairs of electrons at the central atom.
What’s The Difference Between A Tetrahedron And A Pyramid?
A tetrahedron and a pyramid are both three-dimensional geometric shapes. However, they have distinct differences that set them apart from each other.
A tetrahedron is a polyhedron with four faces, all of which are equilateral triangles. It has four vertices and six edges. The base of a tetrahedron is a triangle, and all its other faces are also triangles. It is a regular solid that has no parallel faces, and all its edges have the same length.
On the other hand, a pyramid is a polyhedron with a polygonal base and triangular faces that meet at a common vertex. It has a base that can be any polygon, such as a square or a triangle, and its other faces are triangles. A square-based pyramid, for example, has a square base and four triangular faces. A pyramid has a single vertex, and its height is the perpendicular distance from the base to the vertex.
To sum up, the main difference betwen a tetrahedron and a pyramid is in their base shape and the number of faces they have. A tetrahedron has a triangular base and four faces, all of which are triangles, while a pyramid has a polygonal base and triangular faces that meet at a single vertex.
Is Tetrahedral Pyramidal Or Planar?
Tetrahedral is not a planar shape. It is a three-dimensional shape that forms a pyramid with a triangular base. The tetrahedron has four vertices, four faces, and six edges. Each vertex of a tetrahedron is connected to three other vertices by three edges, and the angles between these edges are all approximately 109.5 degrees. This makes the tetrahedron a highly symmetrical shape, with all four faces being congruent equilateral triangles. However, unlike planar shapes, the tetrahedron cannot be flattened onto a two-dimensional surface without distortion.
Conclusion
The difference between trigonal pyramidal and tetrahedral shapes lies in the presence of a lone pair of electrons in the central atom. While trigonal pyramidal has one lone pair, tetrahedral has no lone pairs. Additionally, a square-based pyramid cannot be considered a tetrahedron, as it has a square base and triangular faces, while tetrahedrons have a triangular base and equilateral triangular faces. Although a tetrahedral shape is not planar, it can be distorted by increasing the angle between two bonds. understanding the differences between these shapes is important in the study of chemistry and molecular geometry.
