Vicinal dihalides are a type of organic compound that contain two halide groups attached to two adjacent carbon atoms of the same chemical compound. These compounds are also known as alkylene dihalides, and are commonly used in the synthesis of various organic compounds.
The synthesis of vicinal dihalides involves the reaction betwen a halogen and an alkene. This reaction is known as halogenation, and typically takes place in the presence of a solvent such as dichloromethane. The halogenation reaction involves the addition of a halogen to the alkene, resulting in the formation of a halogenated alkane. In the case of vicinal dihalides, the halogenation reaction results in the formation of two halogen atoms attached to two adjacent carbon atoms within the same molecule.
Vicinal dihalides are commonly used in the synthesis of various organic compounds, including pharmaceuticals, agrochemicals, and polymers. One of the most common uses of vicinal dihalides is in the synthesis of unsaturated hydrocarbons, which are used as starting materials for the production of a wide range of organic compounds.
In addition to their use in the synthesis of organic compounds, vicinal dihalides also have a number of industrial applications. For example, they are used as solvents, refrigerants, and fire retardants. They are also used in the production of various plastics and resins, as well as in the manufacture of electronic components such as transistors and semiconductors.
Vicinal dihalides are an important class of organic compounds that have a wide range of applications in various industries. Their unique chemical properties make them useful for a variety of purposes, from the synthesis of organic compounds to the production of industrial materials. As such, they are an important area of study for chemists and researchers around the world.
What Is Geminal And Vicinal Dihalide?
Geminal dihalides and vicinal dihalides are two types of organic compounds that contain halogens.
Geminal dihalides are also known as gem-dihalides or 1,1-dihalides. They are compounds that contain two halide groups attached to the same carbon atom. The term “geminal” refers to the fact that the halide groups are located on the same carbon atom and therefore, are adjacent to each other. Geminal dihalides are typically synthesized by the addition of a halogen molecule to an alkene or alkyne.
On the other hand, vicinal dihalides are also known as vic-dihalides or 1,2-dihalides. They are compounds that contain two halide groups attached to adjacent carbon atoms. The term “vicinal” refers to the fact that the halide groups are located on adjacent carbon atoms. Vicinal dihalides are typically synthesized by the addition of a halogen molecule to an alkene or alkyne fllowed by an elimination reaction to form a vicinal dihalide.
Geminal dihalides have two halide groups on the same carbon atom while vicinal dihalides have two halide groups on adjacent carbon atoms.
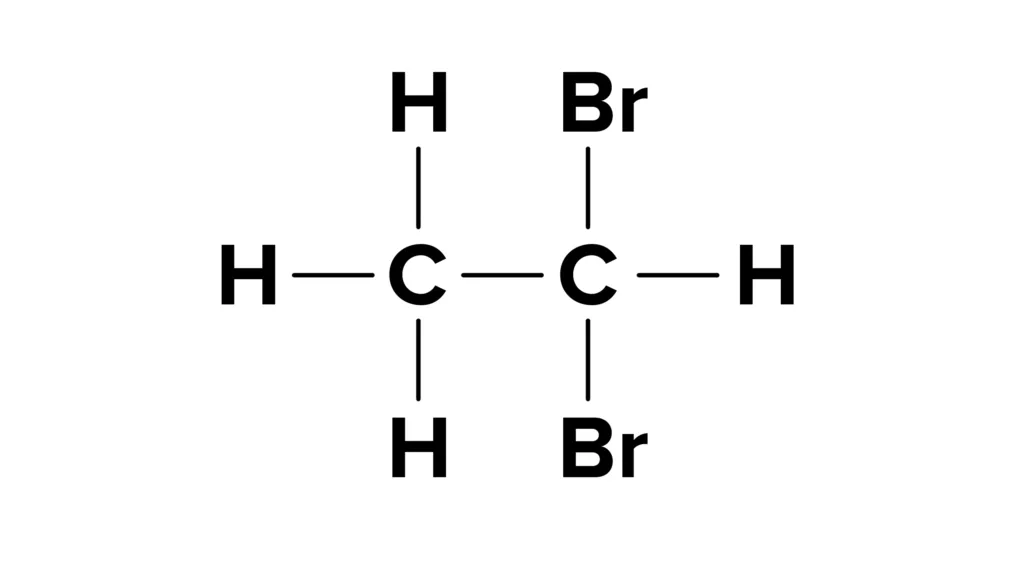
Why Is It Called Vicinal Dihalide?
Vicinal dihalides are so named because they have halogen atoms attached to adjacent carbons in the molecule. The term “vicinal” refers to this proximity or adjacency of the halogen atoms, whch are typically chlorine, bromine, or iodine. The prefix “di-” indicates that there are two halogen atoms in the molecule, and “halide” refers to the fact that they are halogen atoms bonded to carbon atoms. This nomenclature is important in organic chemistry because it provides a systematic way to name and classify compounds based on their structure and composition. Additionally, the term vicinal dihalide is often used to describe a specific type of reaction in which two halogen atoms are added to a carbon-carbon double bond in an alkene. This reaction, known as halogenation, is an important way to synthesize vicinal dihalides and other halogenated organic compounds.
What Is The Other Name Of Vicinal Dihalide?
The other name for vicinal dihalide is alkylene dihalide. This nomenclature is based on the fact that vicinal dihalides have two halogen atoms attached to neighboring carbon atoms in an alkylene chain. The prefix “alkylene” refers to a saturated hydrocarbon chain, and the suffix “-di” indicates that there are two halogen atoms present. Therefore, the term “alkylene dihalide” is a more systematic and precise way of referring to vicinal dihalides. It is commonly used in organic chemistry literature and textbooks to describe this class of compounds.
What Is Vicinal Dibromide?
Vicinal dibromide is a type of organic compound that contains two bromine atoms attached to adjacent carbon atoms in a molecule. The term “vicinal” refers to this adjacency of the two bromine atoms on the molecule. This type of organic compound is commonly prepared through a chemical reaction called bromine addition, whee an alkene is reacted with bromine in the presence of a solvent, such as dichloromethane. The resulting product is a vicinal dibromide, which has a variety of applications in organic chemistry, including as a starting material for the synthesis of other organic compounds. vicinal dibromides are an important class of organic compounds with unique chemical and physical properties that make them useful in a range of applications.
Conclusion
Vicinal dihalides are important organic compounds that contain two halide groups attached to adjacent carbon atoms. These compounds are commonly prepared by the reaction between a halogen and an alkene. Vicinal dihalides have various applications in organic synthesis and are used as intermediates for the synthesis of a wide range of organic compounds. They are also utilized as starting materials for the preparation of polymers, pharmaceuticals, and agrochemicals. The naming system for these compounds is based on the IUPAC nomenclature, with the prefix “alkylene dihalide” being used. vicinal dihalides are significant compounds in both academic research and industrial applications.
