Trigonal planar and trigonal pyramidal are two different molecular geometries that are often encountered in chemistry. The molecular geometry of a molecule is its three-dimensional shape that is determined by the arrangement of the atoms and the lone pairs of electrons around the central atom. The molecular geometry of a molecule can have a significant impact on its physical and chemical properties.
Trigonal planar is a type of molecular geometry that is characterized by a central atom bonded to three other atoms in a flat, triangular arrangement. In trigonal planar geometry, the three bonds are all equal in length and are arranged at 120 degrees to each other. Trigonal planar geometry is commonly found in molecules such as boron trifluoride (BF3) and formaldehyde (H2CO).
Trigonal pyramidal, on the other hand, is a type of molecular geometry that is characterized by a central atom bonded to three other atoms in a pyramid-like arrangement. In trigonal pyramidal geometry, the three bonds are still arranged at 120 degrees to each other, but the presence of a lone pair of electrons on the central atom causes the three bonds to compress slightly, resulting in a bond angle of about 107 degrees. Trigonal pyramidal geometry is commonly found in molecules such as ammonia (NH3) and phosphine (PH3).
The main difference between trigonal planar and trigonal pyramidal geometry is the presence of a lone pair of electrons on the central atom in trigonal pyramidal geometry. This lone pair exerts a little extra repulsion on the three bonding atoms, causing them to compress slightly and resulting in a bond angle that is less than 120 degrees. This compression is not present in trigonal planar geometry, wich lacks a lone pair of electrons on the central atom.
In terms of physical and chemical properties, molecules with trigonal planar geometry tend to be more symmetrical and flat, while molecules with trigonal pyramidal geometry tend to be more asymmetrical and three-dimensional. This can affect their polarity, boiling points, and reactivity. For example, molecules with trigonal pyramidal geometry tend to have a higher boiling point than molecules with trigonal planar geometry, due to the presence of a lone pair of electrons that can form hydrogen bonds with other molecules.
Trigonal planar and trigonal pyramidal are two different molecular geometries that are commonly encountered in chemistry. The presence or absence of a lone pair of electrons on the central atom can significantly impact the three-dimensional shape of the molecule and its physical and chemical properties. Understanding the molecular geometry of a molecule is important for predicting its behavior and interactions with other molecules.
What Makes A Trigonal Pyramidal?
A molecule is said to have a trigonal pyramidal shape when it has four atoms or groups bonded to a central atom, with three of them positioned at the corners of an equilateral triangle and the fourth one located above or below the plane of the triangle. The shape is a result of the repulsion between the bonding pairs of electrons and the non-bonding or lone pair of electrons on the central atom. In the case of a trigonal pyramidal molecule, the lone pair exerts a little extra repulsion on the three bonding atoms, whch causes a slight compression in the bond angle, resulting in a bond angle of 107 degrees. This molecular shape is commonly found in compounds such as ammonia (NH3) or phosphine (PH3).
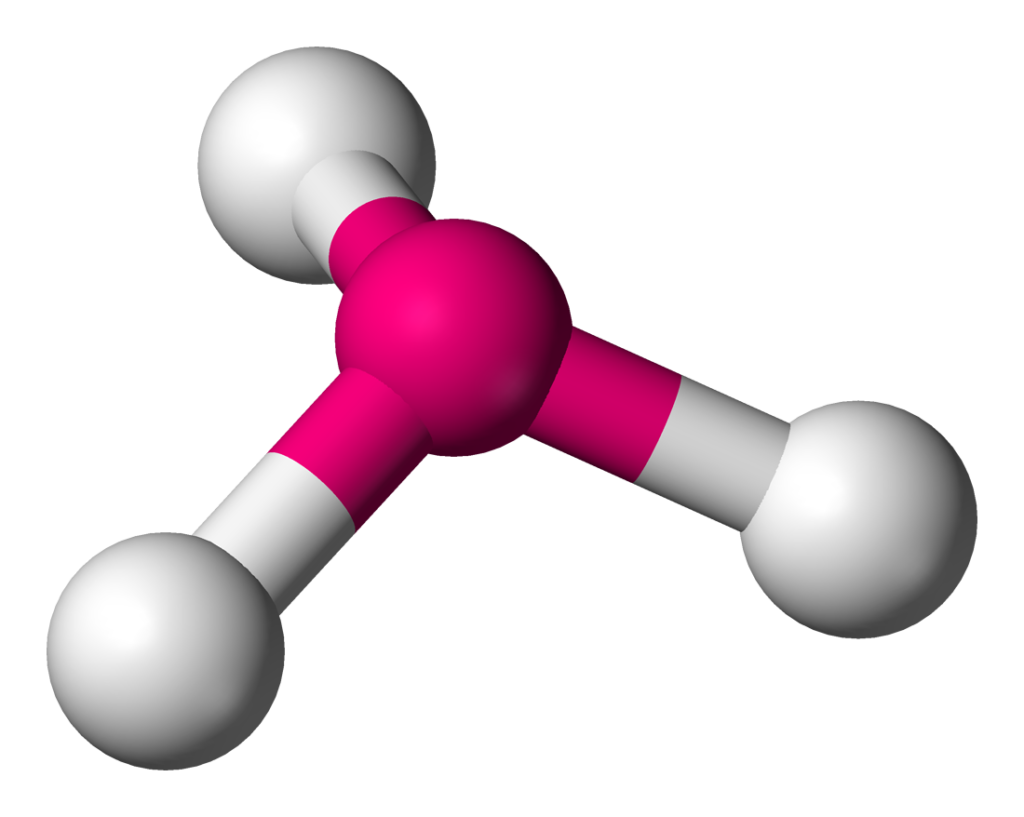
How Do You Know If A Molecule Is Trigonal Pyramidal?
To determine if a molecule is trigonal pyramidal, you need to first look at the central atom and its surrounding atoms. If there is one lone pair of electrons and three bond pairs on the central atom, then the molecule will have a trigonal pyramidal shape. This shape is formed when the bond pairs and the lone pair of electrons repel each other, causing the bond angles to be slightly less than 109.5 degrees. Examples of molecules that have a trigonal pyramidal shape include ammonia (NH3) and phosphine (PH3).
It is important to note that the shape of a molecule is determined by its electron pair geometry, which takes into account both the bond pairs and the lone pairs of electrons. The molecular geometry, on the othr hand, only considers the arrangement of the atoms themselves. In the case of a trigonal pyramidal molecule, the molecular geometry will also be trigonal pyramidal.
A molecule is trigonal pyramidal if it has one lone pair of electrons and three bond pairs on the central atom, resulting in a shape that is pyramidal with bond angles slightly less than 109.5 degrees.
What Is The Difference Between The Triangular Planar And Triangular Pyramid?
Trigonal planar and trigonal pyramidal are two molecular shapes that differ in their geometry and electron configuration. In trigonal planar, the central atom is surrounded by three atoms that are all in the same plane and are arranged in an equilateral triangle. There is no lone pair of electrons on the central atom. This molecular shape is commonly found in molecules such as boron trifluoride (BF3) and formaldehyde (CH2O).
On the other hand, trigonal pyramidal has a central atom that is surrounded by three atoms in a pyramid shape, with one lone pair of electrons on the central atom. The three atoms are arranged in a triangle at the base of the pyramid. This molecular shape is commonly found in molecules such as ammonia (NH3) and phosphine (PH3).
The main difference between these two shapes is the presence or absence of a lone pair of electrons on the central atom. In trigonal planar, thre is no lone pair, while in trigonal pyramidal, there is one. This lone pair affects the geometry of the molecule, causing the atoms to be arranged in a pyramid shape instead of a flat plane.
Trigonal planar and trigonal pyramidal are two different molecular shapes that are distinguished by the presence or absence of a lone pair of electrons on the central atom. Trigonal planar has no lone pair and the atoms are arranged in a flat plane, while trigonal pyramidal has a lone pair and the atoms are arranged in a pyramid shape.
Conclusion
The molecular geometry of a molecule plays a crucial role in determining its physical and chemical properties. Trigonal planar and trigonal pyramidal are two such geometries that are commonly observed in molecules. While both geometries have a triangular arrangement of atoms, the presence or absence of a lone pair of electrons on the central atom distinguishes them. Trigonal planar molecules have no lone pairs, whle trigonal pyramidal molecules have one lone pair of electrons. This slight difference in geometry can significantly impact the molecule’s reactivity, polarity, and other properties. Therefore, understanding the molecular geometry of a compound is essential in predicting its behavior and designing new molecules with specific properties.
