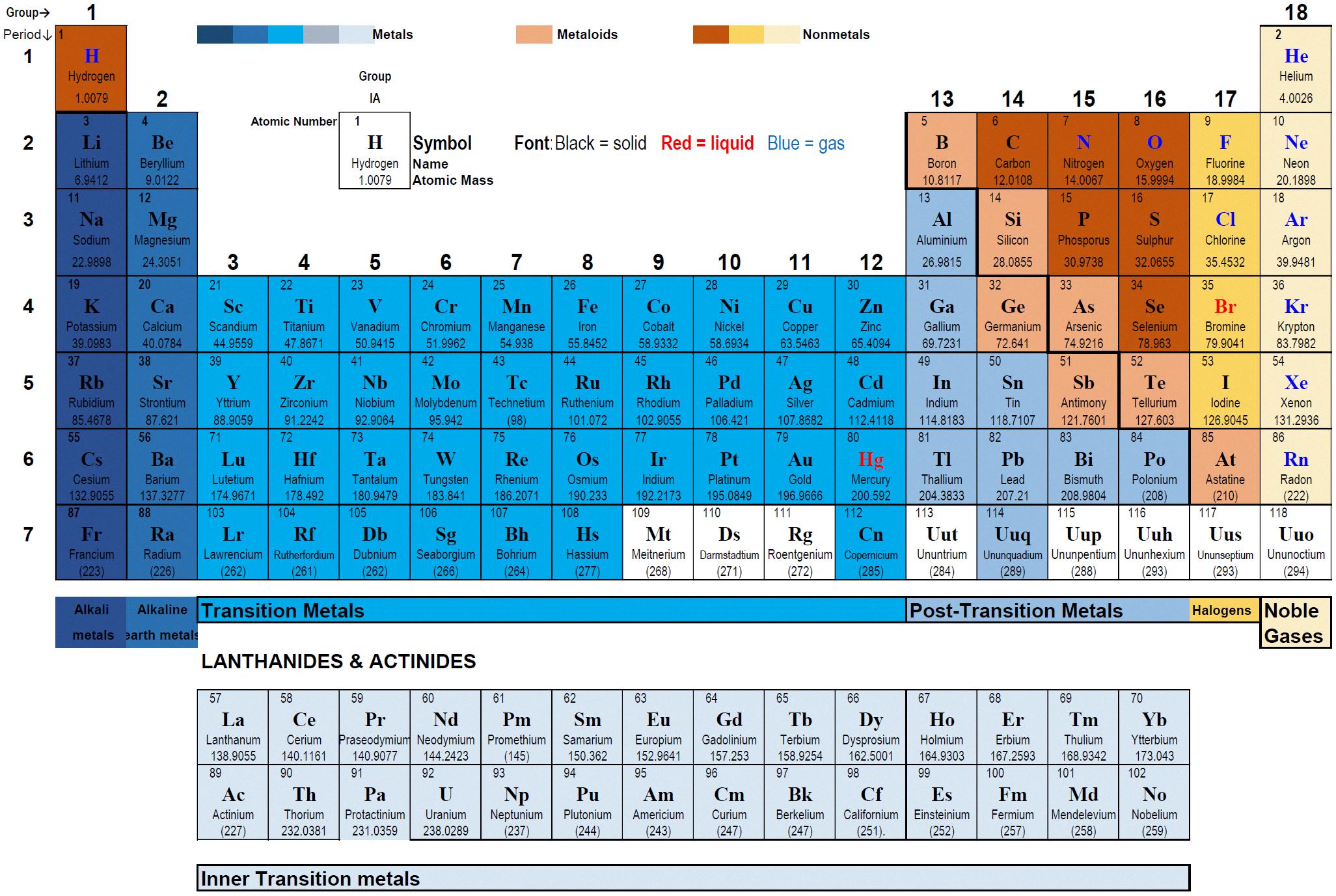
The periodic table is a fundamental tool in the field of chemistry, providing a comprehensive view of all the known elements. However, it can be difficult to determine the properties of an element simply by its position on the table. That’s where the periodic table staircase comes in.
The staircase is a bold line that runs diagonally across the table from boron (B) to astatine (At). The elements that touch or are located to the left of the staircase are typically metals, while those to the right are nonmetals. However, the elements that touch the staircase are known as metalloids, which have properties that are a combination of metals and nonmetals.
Metalloids are unique in their ability to conduct electricity and heat, but not as efficiently as metals. They also have a similar luster to metals, but are generally more brittle and less malleable. They can also form both ionic and covalent compounds, giving them a versatile range of chemical properties.
The staircase also helps us to understand the periodic trends of the elements. For example, as we move from left to right along a period, the elements becme less metallic and more nonmetallic. This is due to the increasing electronegativity of the elements, which causes them to attract electrons more strongly and form more covalent compounds.
In addition, the staircase helps to distinguish between the groups of elements on the periodic table. The elements located in groups 1, 2, and 13-18 are typically metals, while the elements in groups 14-16 are metalloids, and the elements in group 17 are nonmetals.
While the staircase is a useful tool for understanding the properties and trends of the elements, it is important to note that there are exceptions to the general rules. For example, hydrogen (H) is located on the nonmetal side of the staircase, despite having metallic properties under certain conditions.
The periodic table staircase is a crucial element in our understanding of the elements and their properties. It helps to distinguish between metals, nonmetals, and metalloids, and provides insights into the periodic trends of the elements. By understanding the significance of the staircase, we can gain a deeper appreciation for the complexity and diversity of the elements that make up our world.
What Is The Staircase On The Periodic Table Called?
The zigzag line that separates metals from nonmetals on the periodic table is commonly referred to as the “staircase”. This line runs diagonally from the upper left corner to the lower right corner of the table, and elements that touch or are close to the line are knon as metalloids. Metalloids are a small group of elements that exhibit both metallic and nonmetallic properties. The periodic table is also organized into vertical columns called “groups” or “families” and horizontal rows called “periods”. Each element’s position in the table provides valuable information about its atomic structure, chemical behavior, and physical properties.
Why Is The Staircase In The Periodic Table Important?
The staircase present in the periodic table is of great importance as it serves as a divider between metals and non-metals. This staircase is also known as the “zigzag line” or “diagonal line” and is composed of elements called metalloids.
The placement of an element above the staircase indicates that it possesses more metallic properties, whereas elements below the staircase have more non-metallic properties. The metalloids, which are located on the staircase, have properties that are intermediate between metals and non-metals.
This divider helps to classify elements based on their physical and chemical properties, such as their conductivity, reactivity, and melting points. For instance, metals are typically good conductors of electricity and heat, whereas non-metals are poor conductors. This classification of elements based on their properties helps scientists predict the behavior of elements, and use them for varios purposes, such as in industry, technology, and medicine.
The staircase in the periodic table serves as an essential tool for understanding the properties and behavior of elements, and helps to classify them based on their metallic and non-metallic properties.
What Are The Elements Along The Staircase?
The elements along the staircase are called metalloids. These elements possess properties of both metals and nonmetals. They are located along the diagonal line that separates the metals from the nonmetals in the periodic table. Some of the commonly known metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. The metalloids have unique physical and chemical properties that make them useful in various industrial applications.
Where Is The Staircase On The Periodic Table?
The staircase on the periodic table is located at the right-hand side of the table, separating the metals from the nonmetals. It starts at boron (B) and extends down to astatine (At). The staircase is a diagonal line that separates the elements that have properties of both metals and nonmetals, known as metalloids. By identifying the location of the staircase, one can easily determine if an element is a metal, nonmetal, or metalloid. This feature of the periodic table is helpful in predicting the chemical behavior of elements and is widely used in chemistry education and research.
Conclusion
The staircase on the periodic table is a crucial element that helps us classify the elements into metals, nonmetals, and metalloids. It serves as a visual representation of the properties of each element by dividing them based on teir characteristics. The metals are located to the left of the staircase, the nonmetals to the right, and the metalloids touching the line. The presence of the staircase allows us to quickly determine the properties of an element by its location on the table. It is a fundamental tool for chemists and scientists to understand the behavior of elements and their chemical reactions. Therefore, the periodic table staircase is an essential feature that plays a significant role in the study of chemistry.
