When it comes to chemistry and mathematics, understanding how to convert molar to micromolar is an essential skill. It can be a tricky process, but with the right knowledge and understanding of the underlying concepts, anyone can master this important conversion.
From a scientific standpoint, molar and micromolar are two different ways of measuring concentrations. The difference between the two lies in their scale: a molar measurement is much larger than a micromolar measurement. This means that when converting from one to the other, one must multiply by a large number. In this case, one molar is equivalent to one million (1000000) micromolars.
If you are having difficulty understanding the concept behind converting molar to micromolar measurements, here are some helpful tips:
- Start by familiarizing yourself with the terms molar and micromolar: what do they mean and why are they used? Understanding what these terms mean will help you understand the mechanics of converting them.
- Learn how to use scientific notation as this is often used when working with such large numbers as those encountered in this type of conversion. Scientific notation helps break down incredibly large numbers into easier-to-manage chunks that can then be multiplied or divided more easily.
- Understand the relationship between molars and micromolars – namely that one molar is equal to 1 million (1000000) micromolars – so when you’re converting one value into the other, you should always remember that ratio!
- Practice makes perfect! Doing practice conversions will help cement your understanding of both how to convert from mols to microemols as well as doing calculations with scientific notation.
How Do You Convert Micromolar To Moles?
The conversion of Micromolar to moles is done by multiplying the Micromolar vale by 1e-12. This converts the Micromolar to a mole value.
How Do You Convert Molar To NM?
One Molar is equivalent to 1000000000 Nanomolar. To convert Molar to Nanomolar, we just need to multiply the number by 1000000000.
How to Convert Milligrams to Moles and Moles to Milligrams for Elements on the PTOE
How Do You Convert Molar To Mmolar?
Molar and millimolar are both units of concentration. Molar is the more common unit, while millimolar is a thousandth of a molar. To convert from molar to millimolar, divide the molar concentration by 1000. For example, if you have a solution that is 0.5 M in concentration, the millimolar concentration would be 0.5 / 1000 = 0.0005.
How Many Molars Are In A Micromolar?
A micromolar is a very small concentration of molecules in a solution. There are 0.000001 molars in a micromolar.
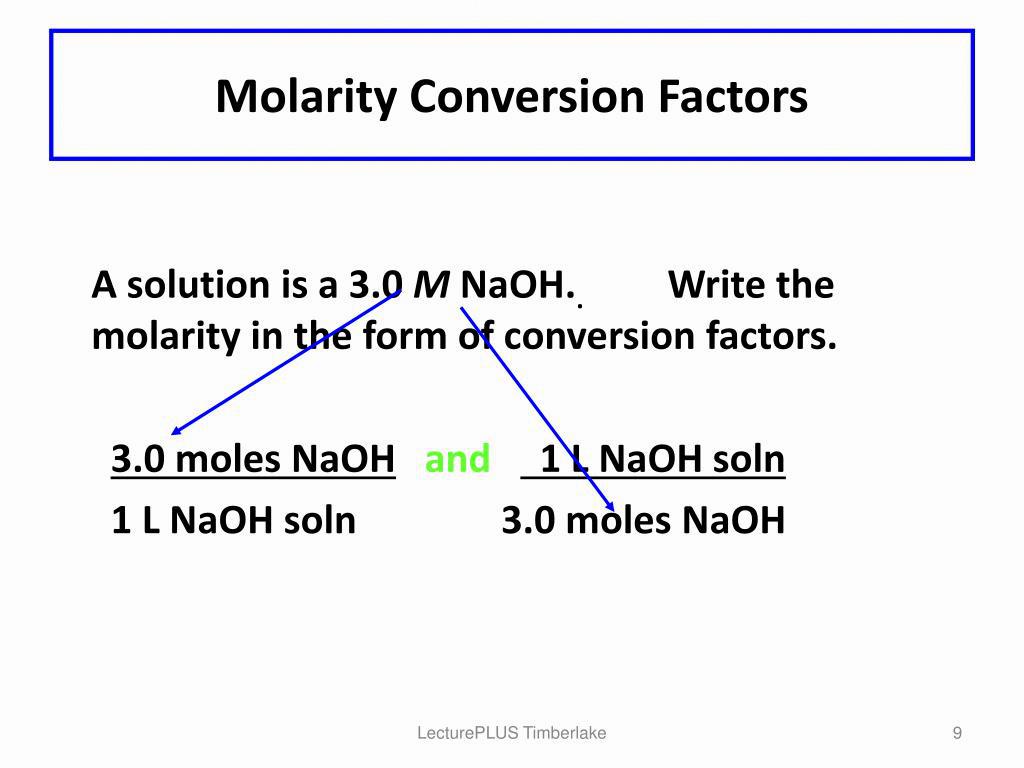
What Is A 1 Molar Solution?
A 1 molar solution (1M) is a solution that contains 1 mole of a compound dissolved in 1 liter of a solution. This means that the concentration of the solution is 1 mol/L or 1M. A 1M solution has a total of 1 mole of solute (the compound that is beng dissolved) and 1 liter of solvent (water).
A molar solution is a way to measure the concentration of a chemical compound in a solution. The concentration of a 1Molar solution is equal to the number of moles of solute per liter of solvent. In other words, a 1Molar solution has a concentration of 1 mol/L.
A molar solution can be used to dilute or concentrate a chemical compound. When diluting a chemical compound, more solvent is added to the solution and less solute is present. When concentrating a chemical compound, less solvent is added to the solution and more solute is present.
The purpose of a molar solution is to provide scientists with a standardized way to measure the concentration of chemicals compounds in solutions. This allows for accurate comparisons between different solutions and makes it easier to see how changes in concentration affect the behavior of chemicals compounds.
How Much Is A Micromole?
A micromole is a unit of measure defined as 10-6 (one-millionth) of a mole. The symbol for micromole is commonly umol or ?mol. Micromoles are commonly used to count the number of photons in a plant grow light system.
What Is Micro Molarity?
A micromolar (?M) is the decimal fraction of a molar, whch is the common non-SI unit of molar concentration. For example, a 2-molar (2 M) solution contains 2 moles of a certain substance in one liter of a liquid or gaseous mixture.
What Micromolar Means?
A micromolar (?M) concentration is a measure of the number of molecules of a particular substance per liter of solution. In oter words, it is a way to express the concentration of a molecule in a given solution. A micromolar solution has one millionth the concentration of a mole per liter.
How Big Is A Nanomole?
A nanomole is one-billionth of a mole. This means that there are 6 followed by 23 zeros (6,000,000,000,000,000) atoms or molecules in a nanomole.
Is MM Millimolar?
Yes, mM is millimolar. Millimolar (mM) is a unit of concentration that equals 1/1,000th of a mole per liter.
How Do You Convert Micromolar To Nanomolar?
Micromolar to Nanomolar conversion is achieved by multiplying the gven micromolar concentration by 1000. For example, a solution with a concentration of 1 micromolar (1 µM) would have a concentration of 1000 nanomolar (1 nM) if converted.
How Do You Convert 1 Molar To Millimolar?
The concentration of a solution is the number of moles of solute per liter of solution. To convert from molar to millimolar, divide by 1000. For example, a 1 molar (1M) solution has 1 mole of solute per liter of solution (1 mM = 0.001M).
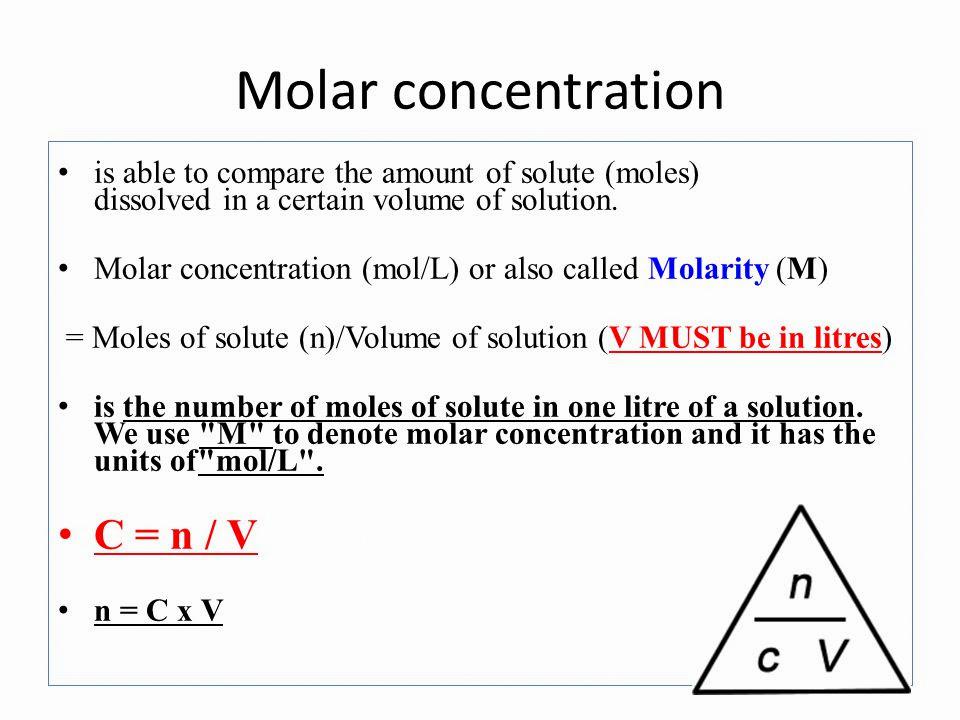
What Is Molar In Chemistry?
In chemistry, molarity (M) is the amount of a substance in a certain volume of solution. Molarity is defined as the moles of a solute per liters of a solution. Molarity is also knon as the molar concentration of a solution.
A mole (mol) is a unit of measurement that represents the amount of a substance that contains as many particles as there are atoms in 12 grams of pure carbon-12.
Therefore, molarity can be calculated by dividing the number of moles of solute by the volume of solution in liters.
How Do You Calculate WV In Chemistry?
The weight/volume (w/v) ratio is the weight of a substance per volume of liquid in wich it is dissolved. This calculation is used to determine the concentration of a solution. The formula to calculate w/v is: wt (g) ÷ vol (mL) = % w/v.
