Molarity is a term used to describe the concentration of a solution. It is an intensive property, which means that it remains constant regardless of the amount of the solution that is being measured. This property is essential in chemistry as it alows for accurate measurements and predictions to be made about the behavior of a solution.
One of the key characteristics of molarity is that it is directly proportional to the amount of solute present in the solution. This means that as the amount of solute increases, the molarity will also increase. Conversely, if the amount of solute decreases, the molarity will decrease as well.
Another important factor that affects molarity is the volume of the solution. Molarity is inversely proportional to the volume of the solution. This means that as the volume of the solution increases, the molarity will decrease, and vice versa.
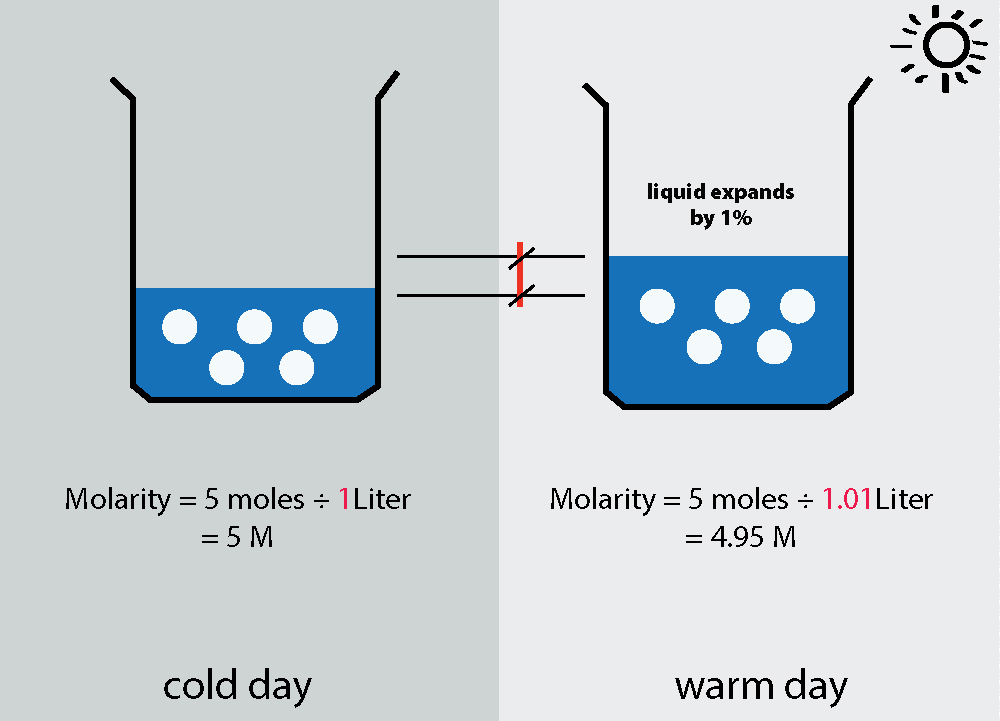
It is important to note that molarity can change with changes in temperature. This is because the volume of a solution changes with temperature. As the temperature of a solution increases, the volume of the solution also increases. This decrease in concentration can be problematic when conducting experiments that require precise measurements.
However, it is important to understand that molality is not affected by changes in temperature. Molality is a measure of the concentration of a solution in terms of the number of moles of solute per kilogram of solvent. Since molality is independent of volume, it remains constant regardless of changes in temperature.
Molarity is a constant property of a solution that is directly proportional to the amount of solute and inversely proportional to the volume of the solution. While it can be affected by changes in temperature, molality remains constant regardless of temperature changes. Understanding the properties of molarity and molality is essential for accurate measurements and predictions in chemistry.
Does Molarity Stay The Same?
Molarity is an intensive property of a solution which remains constant no matter how much solution is present. The molarity of a solution is defined as the number of moles of solute per liter of solution. It is a measure of the concentration of a solution and is independent of the amount of solution present. Therefore, whether you have a beaker filled with a solution or just a spoonful of it, the molarity of the solution woud remain the same. This is because, as long as the number of moles of solute and the volume of the solution remain constant, the molarity of the solution will remain constant. To summarize, the molarity of a solution is an intensive property and, therefore, it does not depend on the amount of solution present.
Does Molarity Change With Volume?
The molarity changes with volume. Molarity is a measure of concentration, which is defined as the amount of solute in a given amount of solution. As the volume of the solution changes, the amount of solute in the solution remains constant, but the concentration changes.
For example, if you dissolve 1 mole of solute into 1 liter of solution, the resulting molarity would be 1 M. If you then add another liter of solvent to the solution, the volume of the solution would increase, but the amount of solute would remain the same. Therefore, the molarity would decrease to 0.5 M.
On the oter hand, if you evaporate some of the solvent from the solution, the volume of the solution would decrease, but the amount of solute would remain the same. Therefore, the molarity would increase.
Molarity is a function of both the amount of solute and the volume of the solution and changes with any changes in either of these variables.
Does Concentration Remain Constant?
At the point of chemical equilibrium, the concentration of both the reactants and products remains constant. This is due to the fact that at equilibrium, the forward and reverse reactions are taking place at the same rate, meaning that the amounts of reactants and products are not changing. Therefore, the concentration of substances in a system at equilibrium will remain constant unless there is a change in the conditions of the system, such as a change in temperature, pressure or the addition of more reactants or products. However, it is important to note that the concentration of substances can fluctuate during the reaction process beore reaching equilibrium.
Why Does The Molarity Of A Solution Remain Constant?
The molarity of a solution is defined as the number of moles of solute per liter of solution. It is dependent on the volume of the solution, which changes with temperature. However, the molarity of a solution remains constant because the volume change is accompanied by an equivalent change in the number of moles of solute present in the solution. This is due to the fact that the amount of solute in a solution is conserved regardess of changes in temperature or volume. Therefore, the molarity of a solution does not change with temperature, but changes with changes in the amount of solute present in the solution.
In contrast to molarity, another concentration unit called molality is independent of volume. It is defined as the number of moles of solute per kilogram of solvent. As a result, molality remains constant with changes in temperature because it is not dependent on the volume of the solution.
The molarity of a solution remains constant because the number of moles of solute present in the solution changes proportionally to the change in volume. This is due to the conservation of the amount of solute in a solution.

Conclusion
Molarity is a constant and intensive property of a solution that is determined by the amount of solute, in moles, dissolved in a solvent by volume and is expressed as moles per liter (mol/L). It is important to note that molarity remains constant regardless of the amount of solution being measured, as it is solely dependent on the amount of solute and the volume of the solution. However, it is important to note that molarity is affected by temperature, as changes in temperature can cause changes in the volume of the solution, which in turn affects the molarity. In contrast, molality is independent of volume and remains constant regardless of temperature changes. Therefore, it is crucial to understand the difference beteen molarity and molality and when to use each measurement in various chemical applications.
