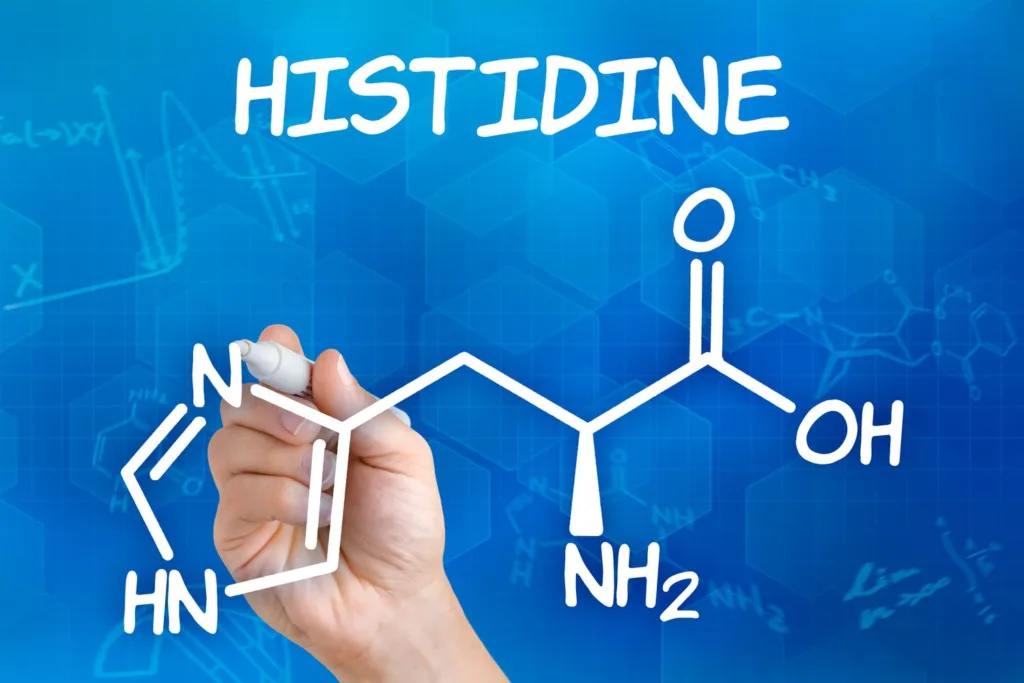
Histidine is one of the 20 amino acids that make up proteins. It is an essential amino acid, meaning that it cannot be synthesized by the body and must be obtained through the diet. Histidine has a unique chemical structure that makes it a versatile amino acid in biochemical reactions.
The side chain of histidine contains an imidazole group, which can be eiher positively charged or neutral depending on the pH of the environment. At pH 7.0, which is close to the physiological pH of the human body, the imidazole group is neutral and does not carry a net charge. This is because the two nitrogen atoms in the imidazole group are involved in resonance, which means that the electrons are distributed evenly between them and there is no net charge.
However, at lower pH values, such as pH 4.0, the imidazole group becomes positively charged. This is because the hydrogen ions in the acidic environment interact with the lone pair of electrons on one of the nitrogen atoms in the imidazole group, causing it to lose its resonance and become protonated. This results in a net positive charge on the histidine molecule.
The ability of histidine to switch between a neutral and a positively charged state makes it an important amino acid in many biochemical reactions. For example, histidine is involved in the catalytic activity of enzymes, where it can act as a proton donor or acceptor depending on the pH of the environment. Histidine residues are also important in protein-protein interactions, where the charge on the imidazole group can facilitate binding to negatively charged molecules.
Histidine is a unique amino acid that can exist in either a neutral or a positively charged state depending on the pH of the environment. At physiological pH, histidine is neutral and does not carry a net charge. However, at lower pH values, the imidazole group becomes positively charged, which can have important implications for its biochemical activity.
Does Histidine Have a Negative Charge at pH 7?
Histidine is an amino acid that has a basic side chain with a nitrogen atom. At a pH of 7.0, which is considered a neutral pH, the side chain of histidine can either be positively or negatively charged depending on the surrounding environment.
However, in most cases, the side chain of histidine is neither positively nor negatively charged at pH 7.0. This is because the pKa value of histidine’s side chain is around 6.0, which means that at a pH of 7.0, the majority of histidine molecules will exist in a zwitterionic form were the nitrogen atom is neither protonated nor deprotonated.
It is important to note that histidine can act as a buffer in biological systems due to its ability to switch between a positively and negatively charged state depending on the pH of the surrounding environment. This property makes histidine a critical amino acid in many biological processes, including enzyme catalysis and protein-protein interactions.
Histidine does not have a negative charge at pH 7.0 but can exist in a zwitterionic form where the nitrogen atom is neither positively nor negatively charged.
The Charge of Histidine at pH 7
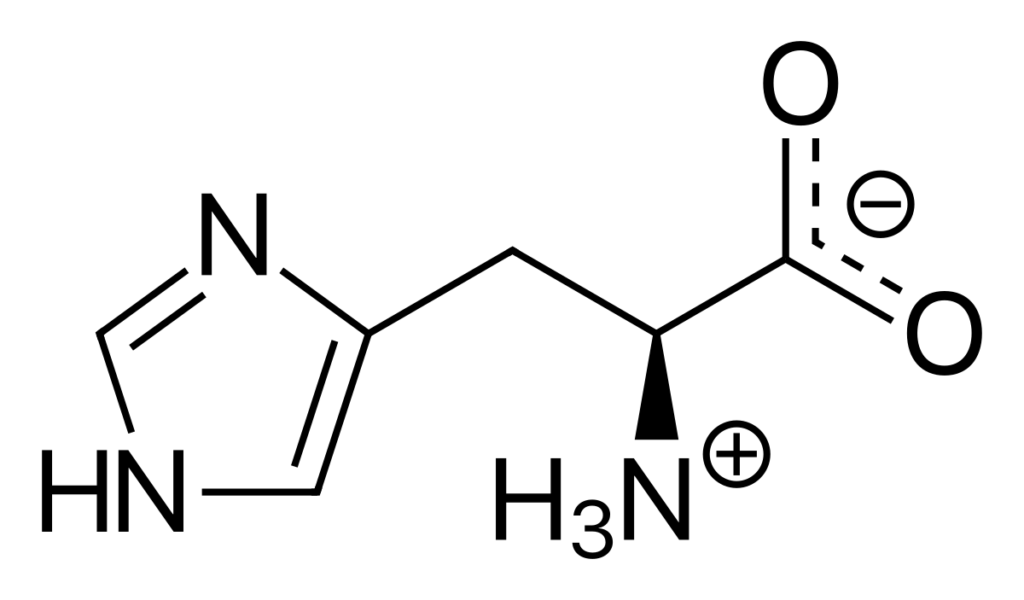
Histidine is an amino acid that contans an imidazole ring, which can act as a proton donor or acceptor depending on the pH of its environment. At physiological pH, which is around 7.4, histidine exists in a zwitterionic form, meaning that it has both a positively charged amino group and a negatively charged carboxylic acid group. Therefore, histidine has no net charge at pH 7, and its overall charge is neutral.
However, in more acidic environments, histidine can become protonated and take on a positive charge. This is because the imidazole ring can act as a weak acid and donate a hydrogen ion (proton) to the surrounding solution. As a result, the positively charged form of histidine, known as the protonated form, is more stable at low pH values.
It’s worth noting that the pKa of histidine’s imidazole group is around 6, which means that at pH values below 6, most of the histidine molecules will be protonated and positively charged. On the other hand, at pH values above 6, most of the histidine molecules will be in the zwitterionic form.
To summarize, histidine is not +1 at pH 7, but rather exists in a zwitterionic form with no net charge. However, its charge can change depending on the pH of its environment, with the positively charged form becoming more prevalent at lower pH values.
Charge of Histidine at Neutral pH
Histidine is an amino acid commonly found in proteins and enzymes. It contains an imidazole group in its side chain, which can act as a proton acceptor or donor depending on the pH of its environment. At neutral pH (pH 7.0), the imidazole group of histidine is partially protonated, resulting in a net charge of zero. This means that histidine is considered a neutral amino acid at this pH.
It is important to note that the charge of histidine can vary depending on the pH of its environment. At lower pH values, the imidazole group becmes fully protonated, resulting in a positively charged histidine molecule. Conversely, at higher pH values, the imidazole group becomes deprotonated, resulting in a negatively charged histidine molecule.
At neutral pH, histidine is a neutral amino acid with a net charge of zero.
Is Histidine Positively Charged at pH 4?
Histidine is a protonated amino acid, which means that at low pH values, it carries a positive charge. At pH 4, the alpha-amino nitrogen and one nitrogen of the imidazole ring are protonated, and their lone pairs are not involved in resonance, allowing them to carry a positive charge. The carboxyl group, on the other hand, remains neutral sice it is already protonated at this pH. Therefore, the net ionic charge on histidine at pH 4 is +2.
It’s important to note that the charge on histidine can vary with changes in pH. At higher pH values, the imidazole ring can become deprotonated, resulting in a neutral charge on the molecule.
Understanding the charge on amino acids like histidine is crucial for understanding their role in protein structure and function.
Conclusion
Histidine is one of the twenty common amino acids that play a crucial role in protein structure and function. Its unique imidazole side chain makes it stand out from oher amino acids, as it can be positively charged, polar, or neutral depending on the pH of the environment. This versatility allows histidine to participate in a wide range of biochemical reactions, including catalysis and metal binding. Moreover, histidine is essential for many biological processes, such as the immune response, oxygen transport, and neurotransmitter synthesis. Therefore, understanding the properties and functions of histidine is of utmost importance for scientists and researchers in fields such as biochemistry, biophysics, and medicine.
