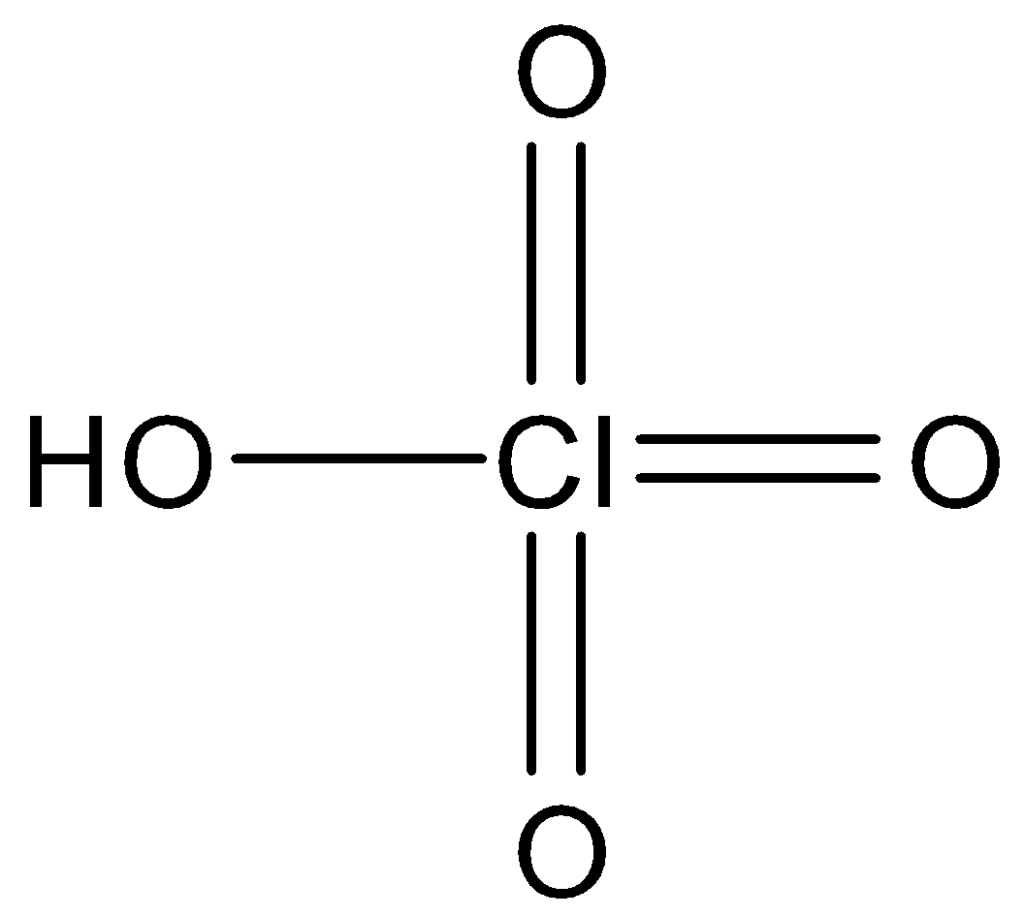
HClO4, also kown as perchloric acid, is a strong acid widely used in chemical laboratories. It is a colorless, odorless, and highly reactive liquid that can dissolve a wide range of substances, including metals, non-metals, and organic compounds. HClO4 is a highly corrosive substance and requires proper handling and storage to avoid accidents.
The strength of an acid depends on its ability to donate a proton (H+) to a water molecule, forming a hydronium ion (H3O+). The more easily an acid can donate a proton, the stronger it is. In HClO4, the oxygen atom attracts the electron density of the hydrogen atom, making the O–H bond weaker and more prone to dissociation. This results in a highly acidic solution with a low pH value.
HClO4 is a stronger acid than HBrO4 due to the electronegativity difference between the halogens. The chlorine atom is more electronegative than the bromine atom, which means it attracts the electron density of the oxygen atom more strongly. This results in a weaker O–H bond in HClO4 compared to HBrO4 and makes it easier for HClO4 to donate a proton.
HCl is a weaker acid than HBr due to the same electronegativity difference between the halogens. The hydrogen atom in HCl is less prone to dissociation than in HBr due to the weaker O–H bond caused by the lower electronegativity of chlorine. This makes HCl less acidic than HBr.
In addition to its use in laboratory experiments, HClO4 is also used in the production of rocket fuels, fireworks, and explosives. It is a highly reactive substance and requires careful handling to avoid accidents. HClO4 is also a strong oxidizing agent and can react violently with combustible materials, causing fires or explosions.
HClO4 is a strong acid due to the weak O–H bond caused by the high electronegativity of the chlorine atom. It is a highly reactive substance that requires proper handling and storage to avoid accidents. Understanding the properties of HClO4 is crucial for its safe and effective use in chemical reactions and industrial processes.
The Strength of Hydrochloric Acid (HClO4)
Hydrochloric acid (HCl) is a strong acid, but what abot HClO4? HClO4 is also a strong acid, and in fact, it is one of the strongest acids known. The reason for this lies in the chemical properties of its conjugate base, ClO4-.
When an acid donates a proton (H+ ion), it forms a conjugate base. In the case of HClO4, it donates a proton to form ClO4-. This conjugate base is highly stable due to the presence of four electronegative oxygen atoms bonded to the central chlorine atom. This stability arises from the delocalization of negative charge over the entire ion, making it highly unlikely for the proton to be accepted back by the conjugate base.
This stability of the ClO4- ion makes HClO4 a strong acid, as it can easily donate its proton to water molecules, forming H3O+ ions. In contrast, weaker acids have less stable conjugate bases, which are more likely to accept the proton back and reform the acid.
Furthermore, HClO4 has more electronegative oxygen atoms than HCl, which also contributes to its greater acidity. The more electronegative the atom, the more it attracts electrons towards itself, making it easier to donate a proton.
The strength of HClO4 as an acid is due to the stability of its conjugate base, ClO4-, and the presence of multiple electronegative oxygen atoms in its structure.

Comparing the Strength of HClO4 and HClO Acids
When it comes to comparing the acidity of two compounds, it’s important to understand the concept of conjugate bases. A conjugate base is the species that remains after an acid has donated a proton (H+). The strength of an acid can be determined by the stability of its conjugate base. In general, the more stable the conjugate base, the weaker the acid.
In the case of HClO4 and HClO, both compounds are acids, but HClO4 is a stronger acid than HClO. This is because the conjugate base of HClO4, ClO−4, is a weaker base than the conjugate base of HClO, ClO−.
The reason for this is due to the difference in the resonance stability of the two conjugate bases. The ClO−4 ion has four oxygen atoms wich can delocalize the negative charge through resonance, making it more stable. In contrast, the ClO− ion has only three oxygen atoms, and the negative charge is not spread out as much, making it less stable.
To summarize, HClO4 is a stronger acid than HClO because its conjugate base, ClO−4, is more stable than the conjugate base of HClO, ClO−.
Comparing the Strength of HClO4 and HBrO4 Acids
In Group 17 oxyacids, the acidity of the acid increases with the increase in the electronegativity of the halogen present in the acid. Both HClO4 and HBrO4 are oxyacids and belong to Group 17 of the periodic table. Chlorine and Bromine are two halogens that belong to this group.
HClO4 has a chlorine atom in it, wereas HBrO4 has a bromine atom in it. Chlorine has a higher electronegativity value than Bromine. This means that the electrons in the O-H bond of HClO4 will be more polarized towards the Cl atom than towards the O atom. Therefore, the O-H bond in HClO4 will be weaker compared to the O-H bond in HBrO4.
A weaker bond will make it easier for an acid to donate a proton. Hence, HClO4 will be a stronger acid than HBrO4.
To summarize:
– HClO4 is a stronger acid than HBrO4
– The electronegativity of the halogen present in the oxyacid determines the acidity of the acid
– Chlorine has a higher electronegativity value than Bromine, making the O-H bond weaker in HClO4 than in HBrO4.
Comparing the Strength of H2SO4 and HClO4 Acids
When it comes to determining the strength of acids, several factors come into play. However, one of the most crucial factors is the ability of an acid to donate a proton, which is also known as its acidity. In general, the stronger the acid, the more easily it donates a proton, and the lower its pH value.
In the case of H2SO4 and HClO4, both are strong acids, but HClO4 is generally considered to be the stronger of the two. The reason for this is that the ClO4- ion is a stronger conjugate base than the SO4-2 ion. This means that HClO4 is more likely to donate a proton than H2SO4, making it a stronger acid.
Another way to compare the strength of acids is to look at their dissociation constants (Ka values). The Ka vale measures the strength of an acid in terms of how readily it donates a proton in water. The Ka value of HClO4 is higher than that of H2SO4, which further supports the idea that HClO4 is the stronger acid.
Although both H2SO4 and HClO4 are strong acids, HClO4 is generally considered to be the stronger of the two due to the strength of its conjugate base and its higher Ka value.
Conclusion
HClO4 is a highly acidic compound due to its ability to form a stable conjugate base. The presence of multiple electronegative oxygen atoms also contributes to its acidity. Compared to HBrO4, HClO4 is a stronger Brønsted acid due to the higher electronegativity of chlorine compared to bromine. However, HCl is a weaker acid than HBr due to the weaker O–H bond caused by the electron density being drawn away from the oxygen atom by the higher electronegativity of chlorine. HClO4 is an important compound in many industrial and laboratory applications due to its strong acidic properties.
