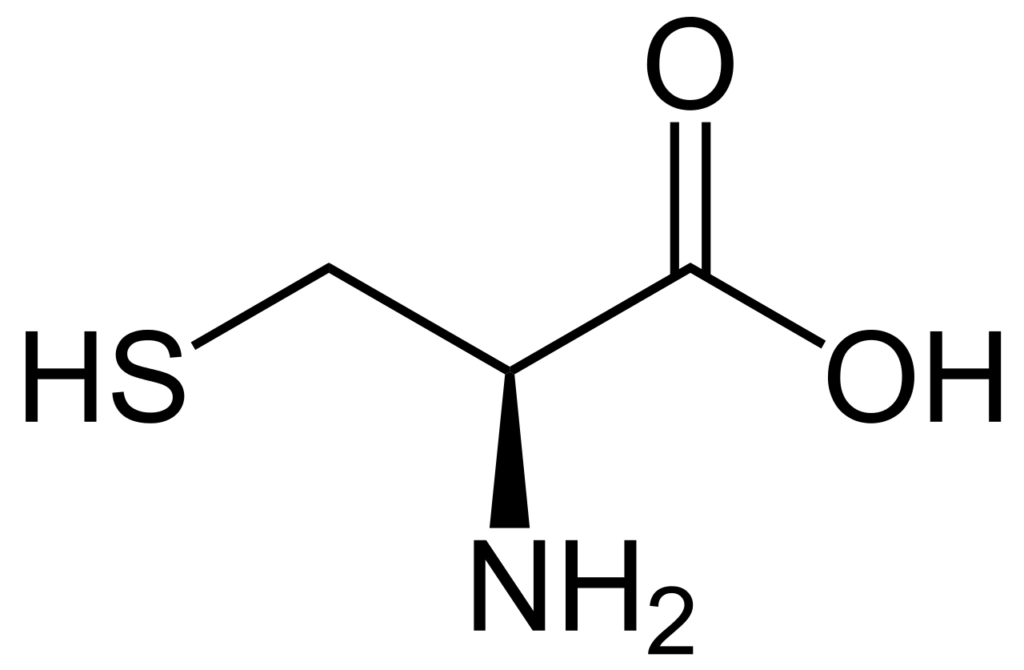
Cysteine is an amino acid that is commonly found in proteins. It contains a thiol group (-SH) in its side chain, whih makes it unique among the amino acids. This thiol group is responsible for the formation of disulfide bonds, which play an important role in stabilizing the structure of proteins.
The question of whether cysteine is polar or nonpolar is a bit tricky. On one hand, the thiol group in cysteine is polar, as it contains an electronegative sulfur atom and a less electronegative hydrogen atom. This makes the cysteine side chain polar overall, with a small positive dipole moment at the hydrogen end.
However, the placement of cysteine in proteins can vary widely. In some cases, it may be exposed to the aqueous environment, making it more polar in nature. In other cases, it may be buried within the protein, shielded from the aqueous environment and interacting primarily with hydrophobic residues. In this context, cysteine may be considered hydrophobic rather than polar.
Recent studies have shed some light on the behavior of free cysteines in proteins. These studies have shown that free cysteines tend to cluster with other hydrophobic residues, such as methionine, tryptophan, and tyrosine. This suggests that free cysteines behave as strongly hydrophobic, and not hydrophilic, residues in proteins.
The question of whether cysteine is polar or nonpolar is not a simple one. While the thiol group in cysteine is polar, the placement of cysteine within a protein can greatly affect its overall polarity. Moreover, the behavior of free cysteines in proteins suggests that they may be more hydrophobic than polar. Further research is needed to fully understand the behavior of cysteine in proteins and its role in protein structure and function.
Polarity of Cysteine on the MCAT
Cysteine is a polar amino acid that is commonly tested on the MCAT. Its polar nature arises from the presence of a thiol (-SH) group in its side chain. This thiol group has a polar covalent bond between sulfur and hydrogen atoms that creates a partial positive charge on hydrogen and a partial negative charge on sulfur, making cysteine a polar amino acid.
In addition to its polarity, cysteine is also unique among amino acids because it contains a sulfur atom in its side chain. This sulfur atom can form a disulfide bond with another cysteine residue, creating a covalent bond that is important for stabilizing protein structure.
It is important to understand the polar nature of cysteine and other amino acids because it affects their interactions with other molecules in biological systems. For example, polar amino acids can form hydrogen bonds with other polar molecules, whle nonpolar amino acids tend to interact with hydrophobic molecules.
Understanding the polarity of amino acids like cysteine is an important concept for success on the MCAT and in understanding biological systems.
Is Cysteine a Polar Amino Acid?
Cysteine is a naturally occurring amino acid that contains a thiol group (-SH) on its side chain. Due to this unique chemical structure, cysteine is classified as a polar amino acid. Polar amino acids are hydrophilic, meaning they are attracted to water molecules and are soluble in aqueous solutions. Cysteine is able to form hydrogen bonds with water molecules, which contributes to its solubility.
However, the polarity of cysteine can vary depending on its location within a protein. When cysteine is part of the protein’s interior, it is often buried and shielded from the surrounding solvent. In this context, cysteine can be considered hydrophobic, as it is not exposed to the aqueous environment. Conversely, when cysteine is located on the surface of a protein, it is exposed to water and oter polar molecules and is considered hydrophilic.
It is important to note that cysteine’s classification as a polar amino acid is based on its chemical structure and its interaction with water molecules in solution. The polarity of cysteine within a protein can vary depending on its specific location and the surrounding environment.
To summarize, cysteine is a polar amino acid due to the presence of a thiol group on its side chain. However, its polarity can vary depending on its location within a protein and its interaction with the surrounding environment.
Polarity of Cysteine
Cysteine is an amino acid that is considered polar due to the presence of a sulfur-hydrogen (S-H) bond in its R group. The electronegativity, or the ability to attract electrons, of sulfur is 2.58, while that of hydrogen is 2.2. This difference of 0.28 results in a polar covalent bond btween the sulfur and hydrogen atoms.
In a polar covalent bond, the electrons are not shared equally between the two atoms. In the case of the S-H bond in cysteine, the sulfur atom attracts the shared electrons more strongly than the hydrogen atom, resulting in a small positive dipole moment at the hydrogen end of the bond. This means that the R group of cysteine is polar, as it has a partial positive charge at the hydrogen end of the S-H bond.
Polarity is an important property of amino acids, as it affects their solubility and interactions with other molecules. Polar amino acids, such as cysteine, tend to be hydrophilic, or water-loving, and are often found on the surface of proteins, where they can interact with water molecules and other polar molecules. Nonpolar amino acids, on the other hand, tend to be hydrophobic, or water-fearing, and are often found buried in the interior of proteins, away from water and other polar molecules.
Cysteine is considered polar due to the presence of a sulfur-hydrogen bond in its R group, which results in a small positive dipole moment at the hydrogen end of the bond. This property makes cysteine hydrophilic and important for interactions with other polar molecules in proteins.
Is Cysteine Polar or Hydrophobic?
Cysteine is an amino acid that cntains a thiol (-SH) group in its side chain. The polarity of cysteine has been a topic of debate in the scientific community, as its thiol group can act as both a hydrogen bond donor and acceptor, which are properties of polar residues. However, recent studies have shown that free cysteine residues in proteins behave more like hydrophobic residues than polar ones.
Cysteine’s hydrophobicity can be attributed to the fact that its thiol group tends to form disulfide bonds with other cysteine residues, which can lead to the formation of hydrophobic clusters in proteins. Additionally, the thiol group can undergo oxidation to form a disulfide bond, which further enhances its hydrophobicity.
In a study published in the Journal of Molecular Biology, the authors analyzed the distribution of cysteine residues in protein structures and found that cysteine residues tend to be located in hydrophobic regions of proteins, along with other hydrophobic residues such as Met, Trp, and Tyr. These residues were clearly separated from polar residues such as Ser and Thr, which were located in polar clusters.
While the thiol group in cysteine does possess some polar properties, free cysteine residues in proteins behave more like hydrophobic residues, and are typically located in hydrophobic clusters in protein structures.
Conclusion
Cysteine is a unique amino acid residue that is often found in functional sites of proteins. Although it is one of the least abundant amino acids, its presence is crucial for protein stability and function. The R group of cysteine contains a thiol group, whih makes it polar and gives it a small positive dipole moment at the hydrogen end. However, despite its polar nature, cysteine is often buried in proteins and behaves as a hydrophobic residue, similar to other aromatic amino acids like tryptophan and tyrosine. This suggests that the hydrophobicity of cysteine plays an important role in protein folding and stability. cysteine is a fascinating amino acid with unique properties that make it essential for many biological processes.
