The carboxyl group is a functional group found in a variety of organic compounds, including amino acids, fatty acids, and carboxylic acids. This group consists of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom. The carbonyl group is a polar group that contains a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom.
The hydroxyl group, on the other hand, is also polar, containing a partial positive charge on the hydrogen atom and a partial negative charge on the oxygen atom. When combined, the carbonyl and hydroxyl groups create a highly polar carboxyl group.
Due to its polar nature, the carboxyl group is highly soluble in water and other polar solvents. This makes carboxylic acids, wich contain one or more carboxyl groups, very useful in a variety of applications.
In addition to its solubility, the carboxyl group also participates in hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom attached to an electronegative atom (such as oxygen) is attracted to another electronegative atom in a nearby molecule. This results in a strong intermolecular bond that can significantly affect the physical and chemical properties of a compound.
The carboxyl group is an important and highly polar functional group found in many organic compounds. Its polarity and ability to participate in hydrogen bonding make it useful in a variety of applications, from pharmaceuticals to polymers.
Polarity of a Carbonyl
Carbonyl is a polar functional group because it has a partial positive charge on the carbon atom and a partial negative charge on the oxygen atom. The oxygen atom is more electronegative than the carbon atom, which leads to the unequal sharing of electrons between them. This unequal sharing of electrons creates a dipole moment, which makes carbonyl polar.
Polarity is an important property of functional groups because it influences teir behavior in various chemical reactions. Polar groups tend to interact more strongly with other polar groups or charged particles, while nonpolar groups tend to interact more weakly with polar groups or charged particles.
It is worth noting that the polarity of a functional group can be influenced by its surroundings. For example, the polarity of a carbonyl group can be enhanced or reduced depending on the nature of the adjacent atoms or groups. This can have significant implications for the reactivity and properties of molecules containing carbonyl groups.
A carbonyl group is polar due to the unequal sharing of electrons between the carbon and oxygen atoms. Its polarity can have important implications for the behavior of molecules containing this functional group.

Source: youtube.com
Polarity of Carboxylic Acid
Carboxylic acids are polar molecules. The polarity of carboxylic acids arises from the presence of two oxygen atoms, one of which is double-bonded to a carbon atom and the oher is single-bonded to a hydrogen atom. The electronegativity of oxygen is higher than that of carbon and hydrogen, meaning that oxygen atoms attract electrons more strongly than carbon and hydrogen atoms. This results in a partial negative charge on the oxygen atoms and a partial positive charge on the carbon and hydrogen atoms.
In addition to the polarity arising from the presence of oxygen atoms, carboxylic acids also have the ability to participate in hydrogen bonding. Hydrogen bonding occurs when a hydrogen atom attached to an electronegative atom, such as oxygen, nitrogen, or fluorine, is attracted to another electronegative atom in a neighboring molecule. In carboxylic acids, the hydrogen atom attached to the oxygen atom in the hydroxyl group can participate in hydrogen bonding with the oxygen atom in the carbonyl group of another carboxylic acid molecule. This gives carboxylic acids a higher boiling point and melting point than nonpolar compounds of similar molecular weight.
The polarity and ability to participate in hydrogen bonding make carboxylic acids highly soluble in polar solvents and less soluble in nonpolar solvents. The polar nature of carboxylic acids is important in many biological processes, such as the formation of peptide bonds in proteins and the breakdown of fats in the body.
Hydrophobicity of Carboxyl Group
The carboxyl group is a hydrophilic functional group. This group is composed of a carbon atom double-bonded to an oxygen atom and single-bonded to a hydroxyl (-OH) group. The oxygen atom in the carboxyl group is highly electronegative, wich creates a partial negative charge, while the hydrogen in the hydroxyl group creates a partial positive charge. This partial charge distribution makes the carboxyl group polar and capable of forming hydrogen bonds with water molecules.
In biochemistry, the carboxyl group is found in amino acids and some amino acid side chains. In amino acids, the carboxyl group is responsible for the acidic properties of these molecules, making them capable of donating protons in solution. The carboxyl group is also present in fatty acids, which are the building blocks of triglycerides and phospholipids. In these molecules, the carboxyl group is responsible for the acid properties and the solubility in water.
The carboxyl group is hydrophilic and capable of interacting with water molecules and other polar molecules through hydrogen bonding. This property is essential for the function of many biological molecules and is an important concept in biochemistry and biology.
Polarity of Carbonyl
Carbonyl is a polar functional group. It is composed of a carbon atom double-bonded to an oxygen atom. The oxygen atom is more electronegative than the carbon atom, which means that it attracts electrons more strongly. This results in a partial negative charge on the oxygen atom and a partial positive charge on the carbon atom, creating a dipole moment. Therefore, molecules containing the carbonyl group are polar.
It is important to note that carbonyl is not ionic. Ionic compounds are formed when tere is a transfer of electrons between atoms, resulting in the formation of positively and negatively charged ions. In the carbonyl group, there is no transfer of electrons, only a sharing of electrons in a covalent bond.
Compounds containing a carbonyl group have higher melting and boiling points than hydrocarbons containing the same number of carbon atoms. This is because the polar carbonyl group allows for stronger intermolecular forces between molecules, requiring more energy to break apart the molecules in order to change its state. Carbonyl-containing compounds are also more soluble in polar solvents such as water due to their polar nature.
Carbonyl is a polar functional group, but not ionic, and its presence in molecules contributes to their higher melting and boiling points and increased solubility in polar solvents.
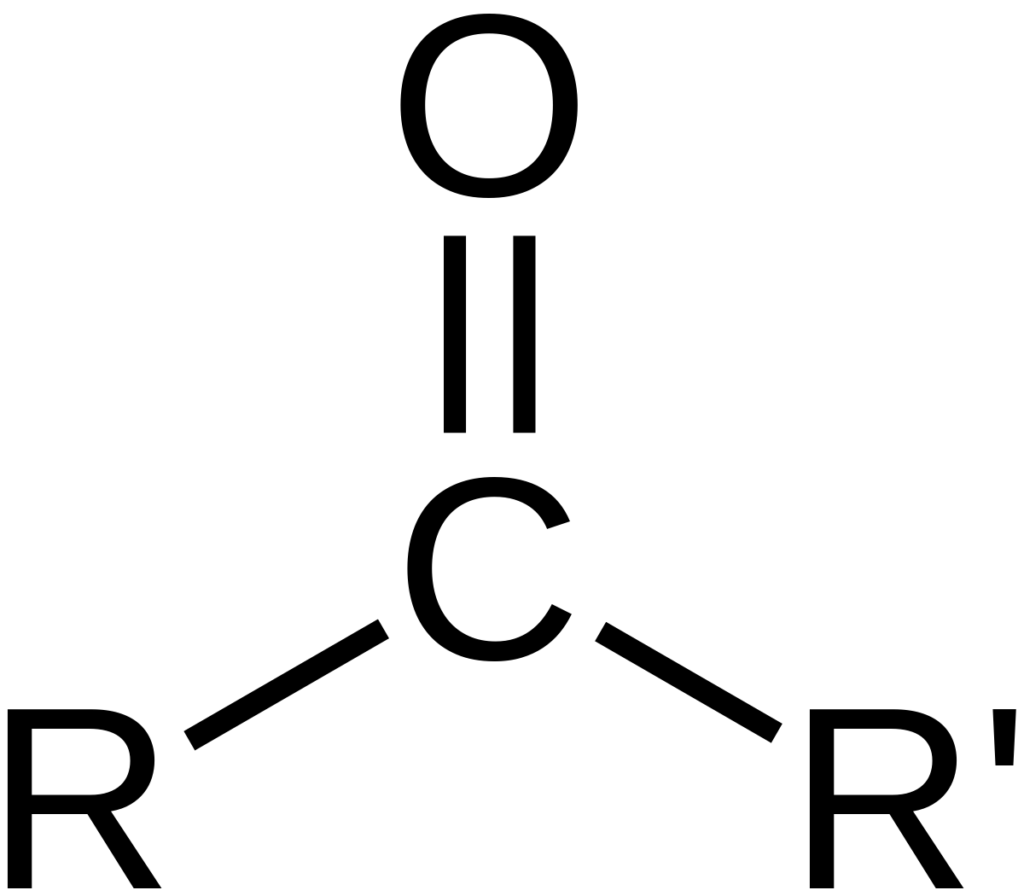
Conclusion
The carboxyl group is indeed a polar functional group due to the presence of two highly electronegative oxygen atoms. This polarity allows carboxylic acid molecules to form hydrogen bonds and interact with other polar compounds, making them highly soluble in water and other polar solvents. The polarity of the carboxyl group also gives rise to other important properties, such as higher melting and boiling points than hydrocarbons of similar size. understanding the polarity of the carboxyl group is crucial in many applications, from biochemistry and pharmaceuticals to organic chemistry and material science.
