When it comes to molecules and their properties, understanding the difference between polar and nonpolar molecules is fundamental. But what does it mean for a molecule to be polar or nonpolar? To answer this question, let’s take a look at the molecule of BF3.
BF3 is a covalent molecule, meaning that the electrons in the molecule are shared equally between the atoms. As such, it has no permanent electric dipole moment, which means that there is no separation of charge. This makes BF3 a nonpolar molecule.
In contrast, NH3 is an ionic molecule with electrons unequally shared between its atoms. This causes there to be a separation of charge, making NH3 a polar molecule with a permanent electric dipole moment.
, BF3 is classified as a nonpolar molecule due to its covalent bond structure and lack of electric dipole moment, while NH3 is classified as a polar molecule due to its ionic bond structure and presence of an electric dipole moment. Understanding these two types of molecules can help us better comprehend their characteristics and behavior in different situations.
Is BF3 (Boron Trifluoride) Polar or Non-polar?
Why BF3 Is Polar Or Nonpolar?
The polarity of a molecule is determined by the presence or absence of a net dipole moment. BF3 has a symmetrical shape and, as a result, the net dipole moment is zero. Therefore, it is nonpolar.
Is BH3 Polar Or Nonpolar?
The Lewis structures for ammonia, NH3, and borane, BH3, indicate that ammonia is polar and borane is nonpolar. The polarity of a molecule is determined by the electronegativity difference between the bonded atoms. The greater the electronegativity difference, the more polar the molecule. In ammonia, the nitrogen atom has a greater electronegativity than the hydrogen atoms, making the molecule polar. In borane, the boron atom has a greater electronegativity than the hydrogen atoms, making the molecule nonpolar.
Why Is BF3 Nonpolar And NH3 Is Polar?
In BF3, the electron pushing power of fluorine is greater than the electron pulling power of boron. This causes more electrons to be pushed towards fluorine, making the molecule more stable overall and less polar. In NH3, on the other hand, the electron pushing power of nitrogen is greater than the electron pulling power of hydrogen. This results in more electrons being pulled towards nitrogen, making the molecule more polar.
What Intermolecular Forces Are Present In BF3?
The intermolecular forces in BF3 are London dispersion forces. These forces arise from the fluctuating electric fields of the atoms in a molecule. They are relatively weak and depend on the proximity of the atoms.
Is BF3 Ionic Or Covalent?
Boron trifluoride is a covalent compound because it forms a strong covalent bond between the boron and fluorine atoms. This bond is formed by sharing electrons between the atoms, whih results in a stable molecule. In contrast, ionic compounds are formed by atoms that exchange electrons to create positive and negative ions.
What Is The Molecular Geometry Of BF3?
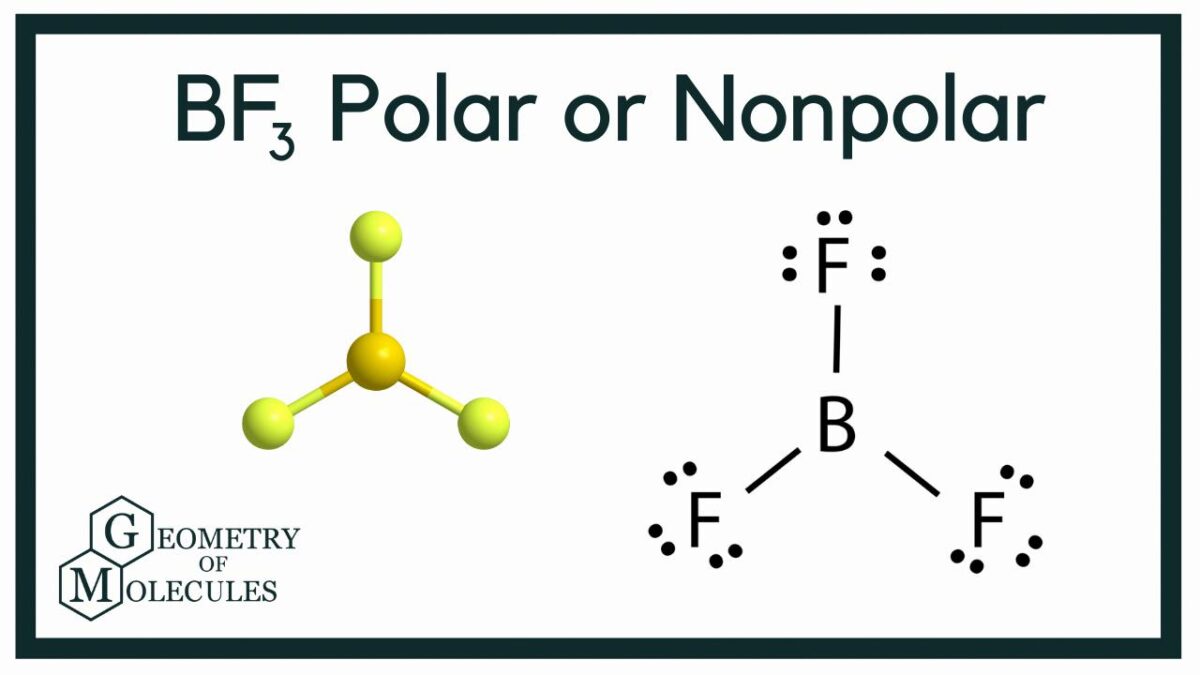
The molecular geometry of BF3 is trigonal planar. This means that the BF3 molecule has three sp2 hybrid orbitals, which are arranged in a triangle with angles of 120 degrees between them.
Why Bcl3 And BF3 Are Non-polar?
The shapes of molecules can play a role in determining polarity. Boron trifluoride (BCL3) and boron trifluoride (BF3) have highly symmetric shapes. This symmetry cancels out the dipole moments of the tree BF bonds, making the resultant dipole moment of the compound equal to 0. Because they are non-polar, BCL3 and BF3 are less likely to interact with other molecules than polar molecules would.
Is BrF3 Polar Or Nonpolar?
The BrF3 molecule is polar becase of the existence of two lone pairs on the central bromine atom. This results in a deformed or twisted trigonal bipyramidal structure, and because the charge distribution on its atoms is non-uniform, the BrF3 molecule is polar in nature.
Is BH3 An Electronegativity?
Yes, BH3 is an electronegativity. The electronegativity of BH3 is 2.04, which is slightly higher than the electronegativities of boron (2.04) and hydrogen (2.20). This means that the bonds in BH3 will be somewhat polarized, with the local dipoles oriented towards the hydrogen atoms.
