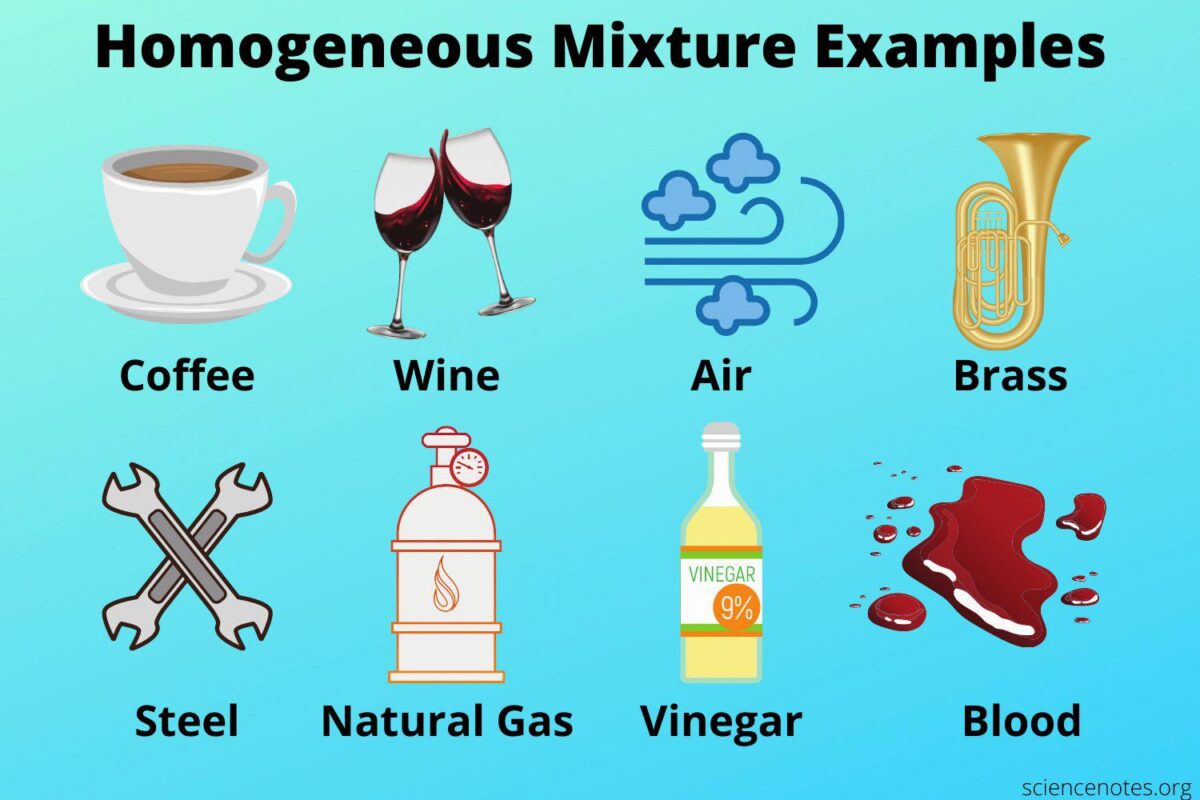
Air is an essential element of life on Earth, and its composition has been a topic of scientific study since the dawn of civilization. In recent years, researchers have sought to understand the nature of air and whether it can be classified as a homogeneous or heterogeneous mixture. The answer depends on what components make up air and how they are distributed.
Air is composed primarily of nitrogen (78%), oxygen (21%), and other gases, such as argon and carbon dioxide (1%). These gases are all in a gaseous state and, when mixed together, form a homogeneous mixture. This means that the composition of air is uniform throughout, meaning that any given sample will have the same ratio of nitrogen to oxygen every time.
A homogeneous mixture does not allow for easy separation of its components. While it is possible to separate out some individual gases from air by using specialised laboratory equipment, this is not practical for most applications. For instance, one cannot separate out the nitrogen from the oxygen in our atmosphere – they are too evenly distributed for any meaningful distinction to be made between them.
Furthermore, due to the small size of its components, air behaves differently than other mixtures such as solutions or suspensions. For example, when two substances are mixed together in a solution or suspension they often form distinct layers that can be seen with the naked eye – this is not true for air because its components are so small and evenly distributed that they cannot be seen without advanced instruments like microscopes or spectrometers.
In summary, air is classified as a homogeneous mixture because it consists primarily of nitrogen and oxygen which are evenly distributed throughout the atmosphere in gaseous form. While these components can be separated with specialist laboratory equipment, it is impossible to do so outside of a lab environment due to their extremely small size and uniform distribution throughout our atmosphere.
Why Is Air A Homogeneous Mixture?
Air is a homogeneous mixture because it is primarily made up of nitrogen and oxygen. These gases are not easily separated or distinguished from one another, so they are considered a homogeneous mixture.
Why Is Air Not A Homogeneous Mixture?
Air is not a homogeneous mixture becaue it contains various gases in specific proportions. Nitrogen and oxygen are the primary gases in air, but there are also other gases present in smaller quantities. These gases can be pollutants, which means they have a negative impact on air quality. When there is too much pollution in the air, it can no longer be considered a homogeneous mixture.
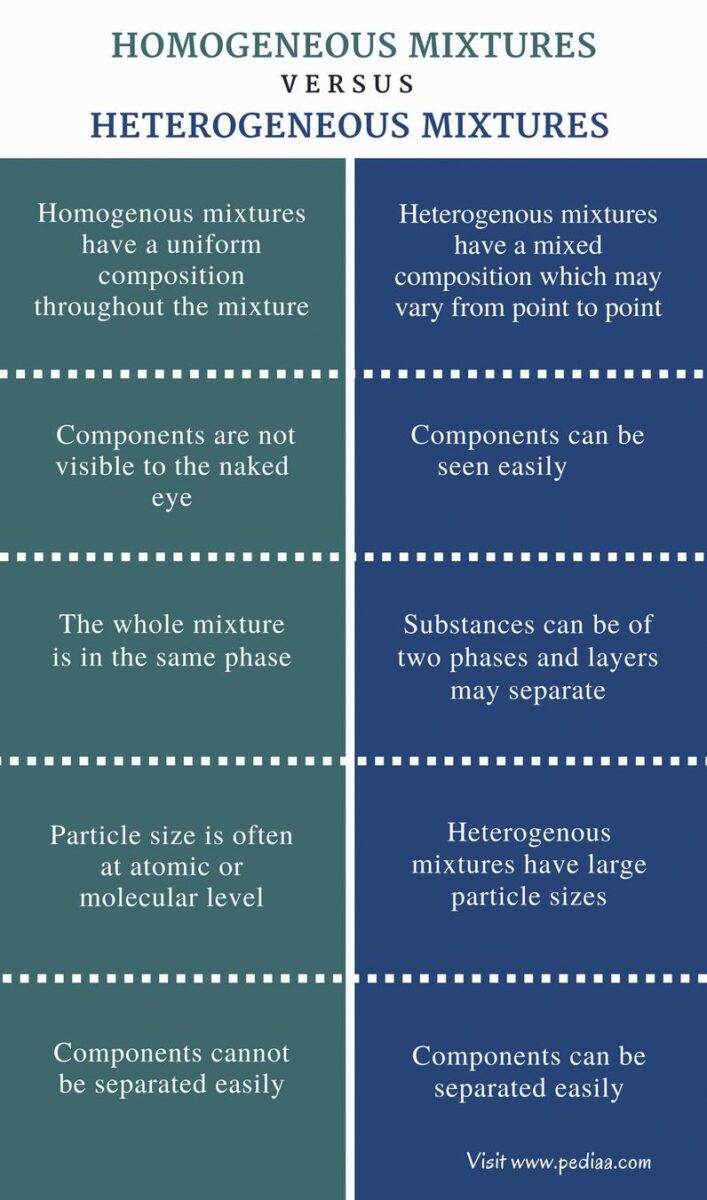
Homogeneous and Heterogeneous Mixture
Is Air Homogeneous Or Compound?
Air is an homogeneous mixture of severl gases. This means that it is a mixture where the different substances (gases) are evenly distributed throughout. It is not a compound because the different substances are not chemically bonded together.
Why Is Air Called Heterogeneous Mixture?
Air is called a heterogeneous mixture because it is made up of different substances that are not evenly mixed together. These substances can be in the form of solid, liquid, or gas particles. The different phases can be separated by physical means, such as filtering or distillation.
Is Air A Heterogeneous Mixture?
Air is a heterogeneous mixture because the components (nitrogen, oxygen, argon, carbon dioxide) are not uniformly distributed. For example, if you took a sample of air from near the ground, it would be different than if you took a sample from high in the sky. Additionally, these components can be separated easily by taking samples. For example, you could use a gas chromatograph to analyze the different gases in air.
What Is Homogeneous Of Air?
The air we breathe is a homogeneous mixture of oxygen, nitrogen, argon, and carbon dioxide. These gases have a uniform composition and are distributed uniformly throughout the air. This means that the concentration of each gas is the same no matter where you are in the air.
Is The Air A Solution?
Yes, the air is a solution. The diagram shows what percentage of air is made up of each gas. There is more nitrogen than any other gas in air, so it is considered the solvent in an air solution.
Is Air A Mixture?
Air is a mixture of gases. The main gases in air are nitrogen, oxygen and carbon dioxide. Other elements in air include argon, neon and helium. Air is a mixture because the different gases are not evenly mixed. They are separated into layers according to their weight. The lightest gases are on the top and the heaviest gases are on the bottom.
Is An Air An Element?
Air is not an element. An element is a substance that canot be broken down into any other substances by chemical means. Air is a mixture of gases, primarily nitrogen and oxygen.
Is The Air In Your House A Homogeneous Or Heterogeneous?
The air in your house is a heterogeneous mixture. This means that it is made up of diferent substances that are not all the same. The air in your house contains both gas and particulate matter. Gas is made up of molecules that are spread out evenly throughout the air. Particulate matter is made up of tiny particles that are not spread out evenly throughout the air.
Which Type Of Mixture Is Air?
Air is a solution. It is a homogeneous mixture of gaseous nitrogen solvent, in wich oxygen and smaller amounts of other gaseous solutes are dissolved.
Is A Air An Element Compound Or Mixture?
Air is a mixture that contans the elements nitrogen, oxygen, and argon, and also the compound carbon dioxide. The proportions of these elements and compounds in air vary depending on location and time of year. Air is not an element, but it is a compound because it contains more than one element.
Why Is Air A Homogeneous Mixture Of Gases?
The gases in the air can be readily differentiated from one another, and the air has a uniform composition of these gases throughout. This is because the air is a homogeneous mixture of gases.
Why Is Air Heterogeneous And Homogeneous?
The air is heterogeneous because it is made up of various gases. The gases can easily be separated from each other. The air is homogeneous because it has one phase of matter.
Is Pure Air An Element Compound Homogeneous Or Heterogeneous?
Pure air is a mixture of gases, which means it is heterogeneous. The different gases in pure air are elements, compounds, or molecules.
