In the field of thermodynamics, the distinction between heat and work is of utmost importance. Heat transfer is the process of transferring thermal energy from one substance to another. The symbol used to represent heat transfer is Q. The uppercase Q is used to represent the total heat transfer, whereas the lowercase q represents the heat transfer per unit mass or unit volume.
In thermodynamics, capital Q represents the total amount of heat transferred into or out of a system. It is the sum of all heat transfer into and out of the system. The unit of measurement for Q is joules (J) or calories (cal).
When it comes to heat transfer, there are three different types: conduction, convection, and radiation. Conduction is the transfer of heat through a material by direct contact. Convection is the transfer of heat through a fluid medium such as air or water. Radiation is the transfer of heat through electromagnetic waves.
The amount of heat transferred through a material by conduction can be calculated using the formula Q = kATΔT, where k is the thermal conductivity of the material, A is the area of heat transfer, T is the temperature difference across the material, and Δx is the thickness of the material.
The amount of heat transferred through a fluid medium by convection can be calculated using the formula Q = hAΔT, where h is the heat transfer coefficient, A is the area of heat transfer, and ΔT is the temperature difference between the fluid and the surface.
The symbol Q is used to represent the total amount of heat transferred into or out of a system. The unit of measurement for Q is joules or calories. The amount of heat transferred through a material by conduction can be calculated using the formula Q = kATΔT, whie the amount of heat transferred through a fluid medium by convection can be calculated using the formula Q = hAΔT. Understanding the difference between heat and work, as well as the different methods of heat transfer, is essential in the field of thermodynamics.
The Effect of Q on Heat
In the field of thermodynamics, there is a clear distinction between heat and work. Heat (Q) and work (W) are both forms of energy transfer, but they are not interchangeable.
Heat is the transfer of thermal energy between two bodies at different temperatures. It flows from the warmer body to the cooler body until they reach thermal equilibrium, which means they are at the same temperature. Heat can be transferred through conduction, convection, or radiation.
Work, on the other hand, is the transfer of energy due to a force acting over a distance. It is the energy required to move an object aginst a force, such as lifting a weight or pushing a box. In thermodynamics, work is often measured in terms of the amount of pressure applied to a system and the resulting volume change.
It is important to note that heat and work are not interchangeable because they have different effects on a system. Heat causes a change in the internal energy of a system, while work causes a change in the system’s volume or pressure.
Q refers to heat, which is the transfer of thermal energy between two bodies at different temperatures. W refers to work, which is the transfer of energy due to a force acting over a distance. Both are forms of energy transfer, but they have different effects on a system and are not interchangeable.
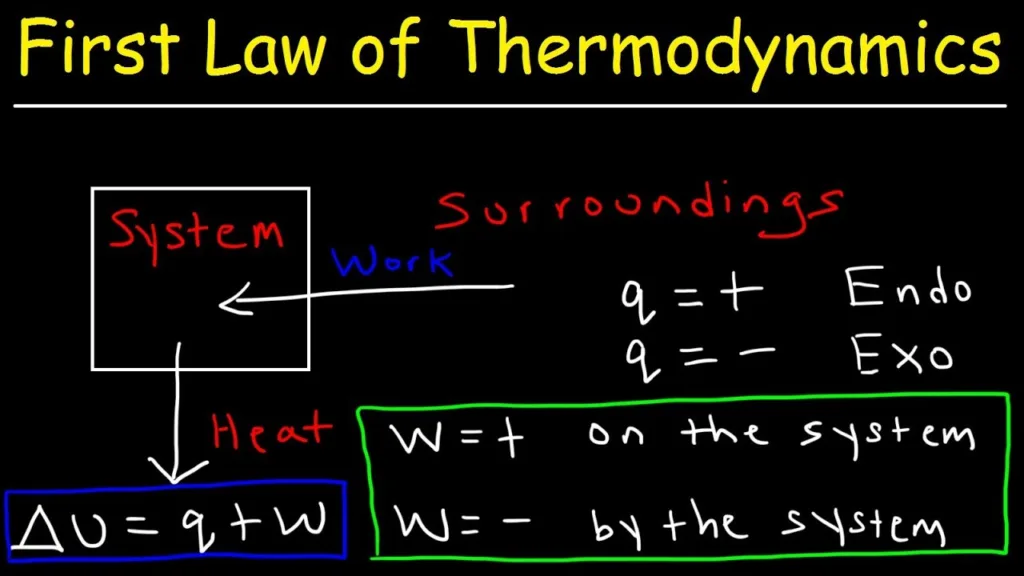
The Meaning of Capital Q in Thermodynamics
In thermodynamics, capital Q represents the net heat transferred into a system. This means that Q takes into account all the heat that is transferred both into and out of the system. Heat transfer is a fundamental concept in thermodynamics, and it refers to the transfer of thermal energy between two objects or systems that are at different temperatures.
In thermodynamics, heat transfer can occur through three different mechanisms: conduction, convection, and radiation. Conduction refers to heat transfer through a solid material, while convection refers to heat transfer through a fluid (such as air or water). Radiation refers to heat transfer through electromagnetic waves.
Capital Q is an important concept in thermodynamics because it helps us understand how energy is transferred into and out of a system. When heat is transferred into a system, it can case the system to increase in temperature, change phase (such as from a solid to a liquid), or undergo other changes. On the other hand, when heat is transferred out of a system, it can cause the system to decrease in temperature, freeze, or undergo other changes.
Capital Q is an essential concept in thermodynamics that helps us understand how heat and energy are transferred into and out of a system. By understanding this concept, we can better understand how different systems and materials behave under different thermal conditions.
The Role of Q in Heat Exchangers
In the context of heat exchangers, Q refers to the amount of heat that is transferred beween two fluids or materials. This heat transfer can occur through multiple mechanisms, including conduction, convection, and radiation.
For conduction heat transfer, Q is calculated using the following formula:
Q = k*A*(T1-T2)/x*t
Where k is the thermal conductivity of the material, A is the area of heat transfer, T1 and T2 are the temperatures of the two materials, x is the thickness of the material, and t is the time of heat transfer.
On the other hand, for convection heat transfer, Q is calculated using the following formula:
Q = h*A*(T1-T2)*t
Where h is the heat transfer coefficient, A is the area of heat transfer, T1 and T2 are the temperatures of the two fluids, and t is the time of heat transfer.
Q is a measure of the amount of heat that is transferred between two materials or fluids in a heat exchanger, and its value depends on the specific heat transfer mechanism that is involved.
Conclusion
The concept of Capital Q refers to the net heat transferred into a system, which is a crucial aspect in the field of thermodynamics. It is important to distinguish between heat and work, as they are two different forms of energy transfer. While a system is in mechanical equilibrium when there is no net force or torque on it, a system is in thermal equilibrium when it is at the same temperature as its environment. The amount of heat transferred in a specific time period is determined by the thermal conductivity, area of heat transfer, temperature, and thickness of the conduction path. Furthermore, the heat transfer coefficient plays a significant role in convection. Therefore, understanding the concept of Capital Q is fundamental for anyone studying or working in the field of thermodynamics.
