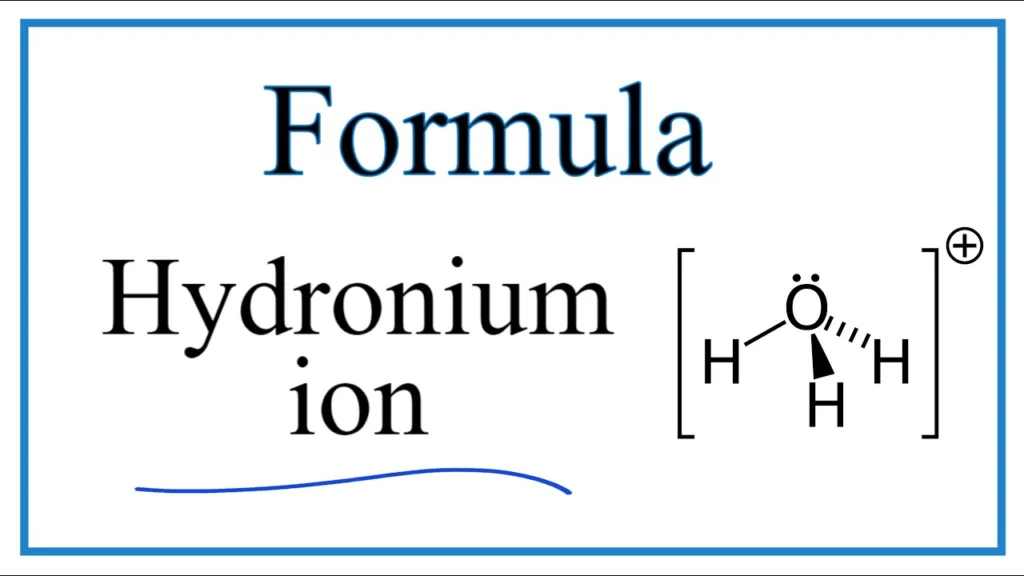
Hydronium ion, with a chemical formula of H3O+, is a crucial component in many chemical reactions. It is formed by the combination of a hydrogen ion (H+) with a water molecule (H2O). The hydronium ion has a trigonal pyramidal geometry, with three hydrogen atoms and one oxygen atom.
One of the most important properties of the hydronium ion is its role in acidity. All acidic aqueous solutions contain protonated water, commonly known as the hydronium ion. When an acid is dissolved in water, it donates a proton to a water molecule, forming a hydronium ion. The strength of an acid is determined by the concentration of hydronium ions in the solution.
Hydronium ions play a vital role in many chemical reactions, including acid-base reactions. In an acid-base reaction, the hydronium ion acts as the acid, donating a proton to a base. This reaction forms a new base and a new hydronium ion.
The formation of hydronium ions can also occur in other chemical reactions. For example, when an alkali metal is dissolved in water, it reacts with water to form a metal hydroxide and hydrogen gas. However, because the reaction is highly exothermic, it can produce enough heat to ionize some of the water molecules, forming hydronium and hydroxide ions.
In addition to its role in acidity, the hydronium ion also has important implications in electrochemistry. When an electric current is passed through a solution containing hydronium ions, the ions can move towards the electrode of opposite charge. This movement of ions is responsible for many important electrochemical processes, including battery charging and electrolysis.
The hydronium ion is an essential component in many chemical reactions. Its role in acidity and electrochemistry makes it a crucial consideration in many applications, from industrial chemistry to biological systems. Understanding the properties and behavior of hydronium ions is essential for anyone working in chemistry or relted fields.
What is the Hydronium Ion Formula?
The hydronium ion, also known as oxonium, is a positively charged ion formed by the combination of a hydrogen ion (H+) with a water molecule (H2O). Its chemical formula is H3O+. The formation of the hydronium ion occurs when a proton (H+) is transferred from an acid to a water molecule, resulting in the formation of the hydronium ion and the conjugate base of the acid.
The hydronium ion has a trigonal pyramidal geometry, wich means that it has a three-dimensional shape with a triangular base and three equal sides that converge at a single point. The oxygen atom of the water molecule has a lone pair of electrons, which gives it a slightly negative charge. The three hydrogen atoms attached to the oxygen are arranged around it to form a tetrahedral shape. The fourth corner of the tetrahedron is occupied by the lone pair of electrons on the oxygen atom.
The hydronium ion is an important species in many chemical reactions, particularly in acid-base reactions. It is the substance responsible for the acidity of aqueous solutions of acids. The strength of an acid is determined by the concentration of hydronium ions in the solution. The higher the concentration of hydronium ions, the stronger the acid.
The formula for the hydronium ion is H3O+, and its formation occurs when a hydrogen ion combines with a water molecule. The hydronium ion has a trigonal pyramidal geometry, and it is an essential component in many chemical reactions, especially in acid-base reactions.
Are H3O+ and H+ the Same?
When discussing the acidity of solutions, the terms H3O+ and H+ may come up. While they are related, they are not exactly the same thing. H+ refers to a hydrogen ion, which is simply a hydrogen atom that has lost its electron. In water (H2O), this hydrogen ion can bond with another water molecule to form H3O+, which is known as the hydronium ion.
So, in a way, H+ can be thought of as the “parent” of H3O+. H3O+ is what happens when the H+ ion bonds with a water molecule. At a molecular level, this means that the water molecule gains a proton, which effectively lowers the pH of the solution.
While H+ is often used as a shorthand for acidity, it’s technically more accurate to use H3O+ when discussing the acidity of aqueous solutions. This is because the H+ ion doesn’t really exist on its own in water – it’s always going to bond with other water molecules to form H3O+.
To summarize: H3O+ and H+ are related, but they are not exactly the same thing. H+ refers to a hydrogen ion, whie H3O+ is what happens when a hydrogen ion bonds with a water molecule. When discussing acidity in aqueous solutions, it’s more accurate to use H3O+ instead of H+.
What is a Hydronium Ion?
An acidic aqueous solution is composed of vaious ions, including the hydronium ion. The hydronium ion is a positively charged ion consisting of three hydrogen atoms and one oxygen atom (H3O+). It forms when a water molecule (H2O) donates a proton (H+) to another molecule, resulting in a hydronium ion and a negatively charged ion.
The hydronium ion is commonly referred to as a protonated water molecule since it is essentially a water molecule with an extra proton. It plays a critical role in acid-base chemistry and is involved in numerous chemical reactions. In acidic solutions, the concentration of hydronium ions is higher than that of hydroxide ions (OH-), resulting in a low pH value.
It is worth noting that the terms “hydronium ion” and “hydroxonium ion” are often used interchangeably to refer to the same ion. The latter term is more commonly used in Europe, while the former is more commonly used in the United States.
The hydronium ion is a positively charged ion consisting of three hydrogen atoms and one oxygen atom that forms when a water molecule donates a proton. It is often referred to as a protonated water molecule and is crucial in acid-base chemistry.
Is the Hydronium Ion H3O+?
H3O+ is indeed a hydronium ion. In chemistry, hydronium ions are formed when a water molecule gains a hydrogen ion (H+) trough protonation. This results in the formation of H3O+ ions, which are also referred to as oxonium ions.
Hydronium ions are important in many chemical reactions and are particularly relevant in acid-base chemistry. They play a crucial role in determining the pH of a solution, as they are often produced when acids dissolve in water.
H3O+ and hydronium ions are interchangeable terms that refer to the same cation, which is formed when a water molecule gains a hydrogen ion.
Conclusion
The hydronium ion, with its chemical formula H3O+, is a crucial component in understanding the properties of acidic aqueous solutions. It is formed by the combination of a H+ ion with an H2O molecule, resulting in a trigonal pyramidal geometry composed of three hydrogen atoms and one oxygen atom. This structure provides a lone pair of electrons on the oxygen, giving it its distinct shape. While the term “hydronium ion” and “H+” ion are oten used interchangeably, the former is technically more accurate as it reflects the molecular level changes occurring in the solution. By understanding the properties and behavior of the hydronium ion, scientists and chemists can better comprehend the behavior of acidic solutions and their impact on various chemical reactions.
