Phosphorus is a chemical element with an atomic number of 15, making it one of the most abundant elements on Earth. This element is part of the nitrogen group, which also includes nitrogen, oxygen, and sulfur. Not only is phosphorus essential for life on our planet, but it also plays an important role in many industrial processes.
At the atomic level, phosphorus has 15 protons in its nucleus and 15 electrons orbiting around it. This gives phosphorus a neutral charge since the number of protons and electrons are equal. As such, it does not have an overall electric charge either positively or negatively charged.
Due to its relatively simple structure, phosphorus is used in a variety of applications throughout many industries. For example, it can be found in fertilizers to help plants grow and thrive; it is used to make matches; and it is even used as an ingredient in some types of food preservatives. It can also be used as a brightener in some paints and dyes, as well as a catalyst in certain chemical reactions.
In addition to its versatility from an industrial standpoint, phosphorus has also become increasingly important for medical purposes in recent decades. For example, this element is often used for imaging studies such as magnetic resonance imaging (MRI) scans due to its ability to absorb energy from radio waves or x-rays and then release that energy again into the surrounding environment at different wavelengths.
Overall, phosphorus proves itself time and time again to be an incredibly versatile element with far-reaching implications both inside and outside the laboratory setting – from helping us better understand our physical world through medical imaging techniques to providing vital nutrients for plants so that we may continue growing our food supply sustainably and efficiently.
How Many Electrons Are Present In Phosphorus?
There are 15 electrons in a phosphorus atom. The atomic number of phosphorus is 15, which means that it has 15 protons and 15 electrons. Phosphorus is a member of the group 5A elements in the periodic table, which means it has five valence electrons. These five valence electrons are the electrons that are available to form chemical bonds with other atoms.
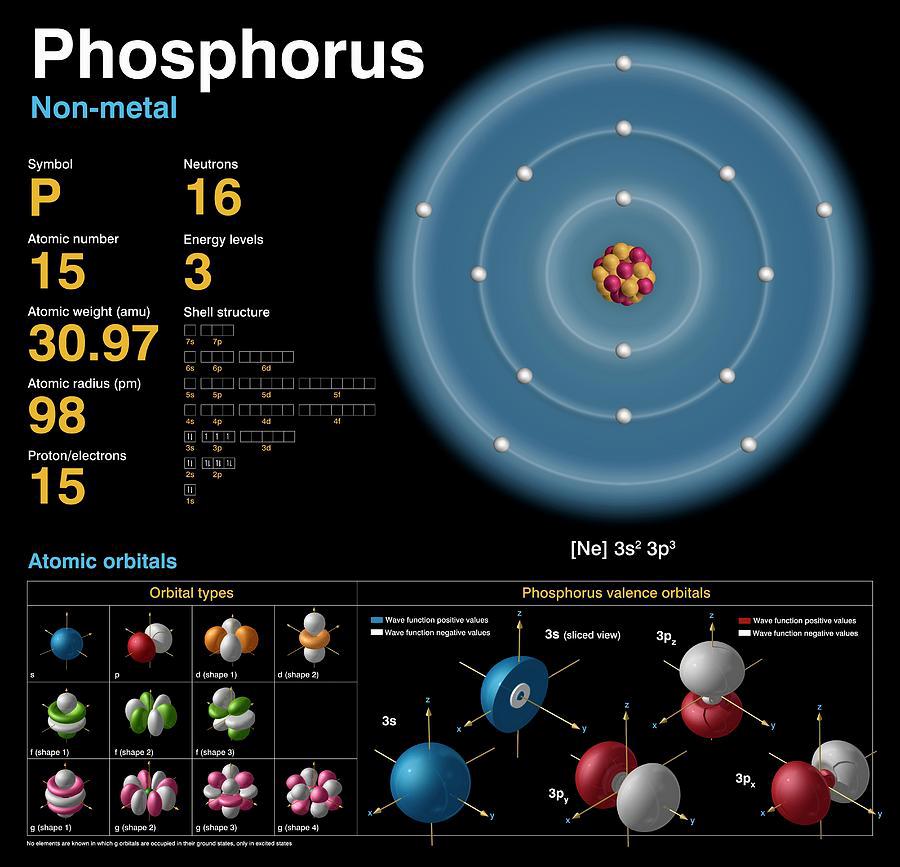
How Many Protons And Electrons Does Phosphorus P Have?
Phosphorus (P) has an atomic number of 15 and therfore has 15 protons in its nucleus. It also has 15 electrons orbiting its nucleus. Phosphorus is electrically neutral because the number of protons equals the number of electrons.
How Many Electrons Does Phosphorus 31 Have?
The isotope phosphorus-31 has 15 protons, 15 electrons, and 16 neutrons.
Does Phosphorus Have 15 Valence Electrons?
Yes, phosphorus has 15 valence electrons. The third energy level of the atom contains five valence electrons. These electrons are availale to participate in chemical reactions.
What Has 15 Electrons And 16 Neutrons?
Phosphorus has 15 electrons and 16 neutrons. It is located on the periodic table in group 5A, which is also knwn as the nitrogen family. Phosphorus is a nonmetal, meaning it does not have the physical properties of a metal. It is a brittle, white solid that is soluble in water. Phosphorus is used in many different applications, including as a fertilizer and in fireworks.
How Many Electrons Does Phosphorus Have In Its Outer Shell?
Phosphorus has five electrons in its outer shell. This is bcause it belongs to Group 5A of the periodic table, which corresponds to elements with a valence of five.
How Many Electrons Does Phosphorus-32 Have?
Phosphorus-32 has 15 protons and 17 neutrons in its nucleus. This isotope of phosphorus has one more neutron than the most common isotope of phosphorus, phosphorus-31. This extra neutron results in a beta emission of 1.709 when the nucleus decays.
How Many Protons Does Phosphorus-32 Have?
The nucleus of phosphorus-32 contans 15 protons and 17 neutrons. This makes phosphorus-32 a neutron-rich isotope of phosphorus.
How Many Electrons Does Phosphorus 33 Have?
Phosphorus 33 has 17 protons, 17 electrons, and 18 neutrons.
