Louis de Broglie was a French physicist who made significant contributions to the field of quantum mechanics. One of his most notable achievements was his theory that electrons, which were previously thought of as particles, also have wave properties.
De Broglie’s theory was based on the idea that if light could behave as both a wave and a particle, then perhaps other particles, such as electrons, could as well. He proposed that electrons should be considered as waves confined to the space around an atomic nucleus. In this way, electron waves could exist only at specific frequencies.
De Broglie concluded that most particles are too heavy to observe thir wave properties. When the mass of an object is very small, however, the wave properties can be detected experimentally. De Broglie predicted that the mass of an electron was small enough to exhibit the properties of both particles and waves.
To test his theory, de Broglie proposed an experiment in which electrons would be scattered from atoms in a crystal. If electrons had wave properties, they would produce an interference pattern similar to the pattern produced by light waves passing through a diffraction grating.
The experiment was conducted, and the results confirmed de Broglie’s theory. The scattered electrons produced an interference pattern, which was characteristic of wave-like behavior and was exhibited by both electrons (i.e., matter) and electromagnetic radiation (i.e., light).
The electron’s wavelike motion is described mathematically by a so-called Bloch wavefunction. Named after the 20th-century physicist Felix Bloch, who was the first to describe the behavior of electrons in crystalline solids, these wavefunctions are complex – that is, they have both real and imaginary components.
De Broglie’s theory that electrons have wave properties was a significant contribution to the field of quantum mechanics. His proposal was based on the idea that if light could behave as both a wave and a particle, then other particles, such as electrons, could as well. The experiment he proposed confirmed his theory and opened up new possibilities for understanding the behavior of matter at the quantum level.
De Broglie’s Conclusion of Electron Wave Nature
Louis de Broglie, a French physicist, proposed that electrons could be considered as wave-like particles in 1923. Prior to this, scientists believed that electrons behaved solely as particles and did not exhibit wave-like behavior. De Broglie suggested that if light could exhibit both particle and wave-like behavior, then perhaps particles could also exhibit wave-like behavior.
De Broglie’s hypothesis was based on the principles of wave-particle duality, whih states that all matter can exhibit both wave-like and particle-like behavior. He suggested that electrons should be considered as waves confined to the space around an atomic nucleus; in this way, electron waves could exist only at specific frequencies. This idea was confirmed by experiments that showed that electrons could be diffracted just like waves.
De Broglie’s work was crucial in the development of the field of quantum mechanics, which studies the behavior of matter at the atomic and subatomic level. It helped to explain the strange behavior of particles at this level, such as the fact that particles can exist in multiple states at the same time. De Broglie’s theory was also important in the development of modern electronics, as it laid the groundwork for the understanding of electron behavior in semiconductors.
De Broglie concluded that electrons have a wave nature by proposing that they could be considered as waves confined to the space around an atomic nucleus. This idea was based on the principles of wave-particle duality and was confirmed by subsequent experiments. De Broglie’s work was instrumental in the development of quantum mechanics and modern electronics.
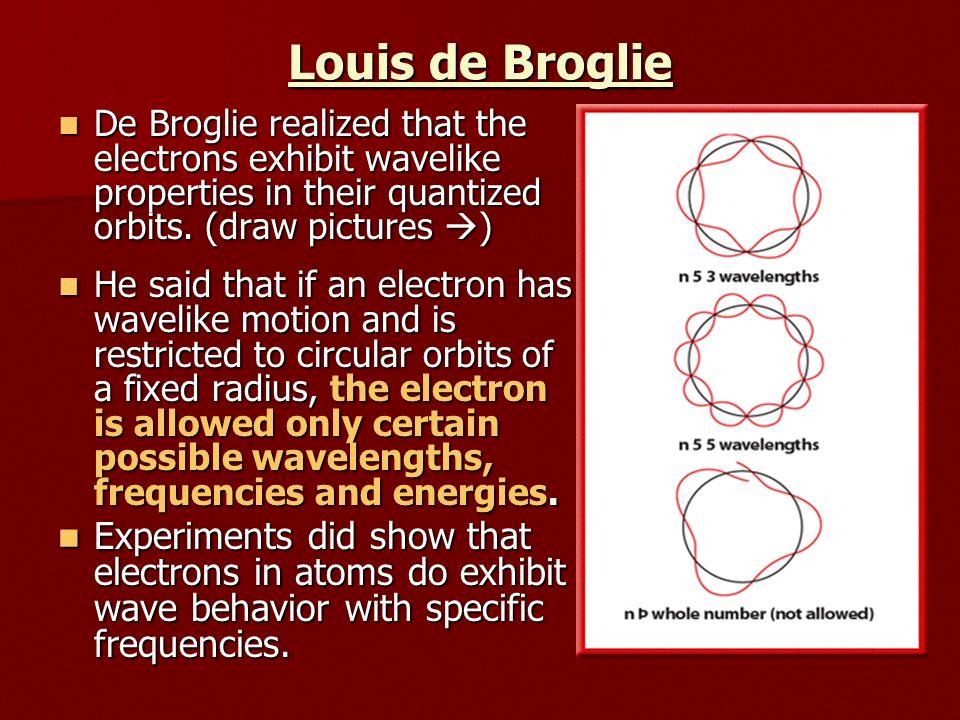
Conclusion of De Broglie’s Theory
Louis de Broglie was a French physicist who proposed the wave-particle duality theory in 1924. According to this theory, particles, such as electrons, can exhibit wave-like properties uder certain circumstances. De Broglie’s conclusion was based on his observation that waves could be described mathematically in the same way as particles. He believed that particles, including electrons, could also be described as waves with a specific wavelength and frequency.
De Broglie’s theory was based on the principles of quantum mechanics, which suggest that the behavior of particles can be described using probability functions. He proposed that the wavelength of a particle is inversely proportional to its momentum. This means that particles with a low mass and high velocity, such as electrons, should exhibit wave-like properties.
De Broglie’s theory was supported by the famous double-slit experiment, which showed that electrons can interfere with each other like waves when passing through a narrow opening. This experiment provided strong evidence for the wave-particle duality theory and helped to establish de Broglie as one of the pioneers of quantum mechanics.
De Broglie’s conclusion was based on his theoretical and experimental work, which showed that particles, such as electrons, can exhibit wave-like properties under certain conditions. His theory revolutionized the field of physics and paved the way for the development of quantum mechanics.
The Wave-Like Properties of Electrons
Electrons, which are subatomic particles that orbit aound the nucleus of an atom, have wave-like properties due to their dual nature as both particles and waves. The concept of wave-particle duality was first proposed by Louis de Broglie in 1924, who suggested that all matter has both wave-like and particle-like characteristics.
One of the key pieces of evidence for the wave-like behavior of electrons comes from the famous double-slit experiment, which was first performed by Thomas Young in 1801 using light waves. In this experiment, a beam of light is passed through two parallel slits, which creates an interference pattern on a screen behind the slits. This pattern is caused by the waves from the two slits interfering with each other, creating areas of constructive and destructive interference.
In a similar way, when electrons are fired at a crystal, they can also create an interference pattern. This is because the electrons can behave like waves, which can interfere with each other to create areas of constructive and destructive interference. This interference pattern is only possible if the electrons have wave-like properties.
The wave-like behavior of electrons is also described by the Schrödinger equation, which is a mathematical equation that describes the behavior of quantum particles. This equation predicts that the position and momentum of a particle cannot both be precisely known at the same time, which is known as the Heisenberg uncertainty principle.
The wave-like properties of electrons are a fundamental aspect of their behavior, which can be observed in a variety of experiments and described by mathematical equations.
The Wave Nature of Electrons
Electrons exhibit wave-like behavior, which is a fundamental aspect of quantum mechanics. The wave nature of electrons is described by their Bloch wavefunction, named after physicist Felix Bloch, who pioneered the study of the behavior of electrons in crystalline solids.
The Bloch wavefunction is a complex function that has both real and imaginary components. It describes the probability of finding an electron in a partcular location in a crystal lattice. The wave nature of electrons is significant in understanding the electronic properties of materials, such as their electrical conductivity and optical properties.
The wave nature of electrons can be visualized using the de Broglie wavelength, which is a property of all matter. According to the de Broglie wavelength, the wavelength of an electron is inversely proportional to its momentum. This means that electrons with higher momentum have shorter wavelengths and exhibit more wave-like behavior.
The wave nature of electrons is critical in understanding many phenomena in modern physics, such as the behavior of electrons in semiconductors and the quantum mechanics of atoms and molecules. It is also essential in the development of technologies such as transistors, lasers, and computer chips.
The wave nature of electrons is a fundamental aspect of quantum mechanics that is described by the Bloch wavefunction. This wave-like behavior is significant in understanding the electronic properties of materials and in the development of modern technologies.
Confirmation of De Broglie’s Hypothesis Regarding the Wave Nature of Electrons
Louis de Broglie’s hypothesis about the wave nature of electrons was confirmed experimentally in 1927 by Clinton Davisson and Lester Germer, in what is now known as the Davisson-Germer experiment. The experiment involved firing electrons at a crystalline nickel target and observing the diffraction patterns created by the scattered electrons. The results showed that the electrons behaved as waves, which was consistent with de Broglie’s hypothesis. This experiment was a crucial step in the development of quantum mechanics and helped establish the wave-particle duality of matter.
Source: slideplayer.com
Confirming the Wave-like Nature of Electrons
The Davisson-Germer Experiment is the experiment that confirmed that electrons have a wave-like nature. This experiment was conducted in 1927 by Clinton Davisson and Lester Germer, who observed the diffraction of electrons by a crystal.
The experiment involved firing a beam of electrons at a nickel crystal and observing the pattern of diffraction that was produced. If the electrons behaved like particles, they woud produce a simple scattering pattern on the detector screen. However, what was observed was a diffraction pattern that was consistent with the behavior of waves.
This observation confirmed the earlier hypothesis of Louis deBroglie that electrons, like other particles, could exhibit wave-like behavior. By demonstrating the wave-particle duality of electrons, the Davisson-Germer Experiment represented a major step forward in the development of quantum mechanics.
The Davisson-Germer Experiment confirmed the wave-like nature of electrons by observing the diffraction of electrons by a crystal. This experiment demonstrated the wave-particle duality of electrons and represented a major breakthrough in the development of quantum mechanics.
Proof That Electrons Are Waves
Louis de Broglie, a French physicist, is credited with proving that electrons have a wave nature. He proposed the theory of wave-particle duality, which suggested that all matter has both wave-like and particle-like properties. In 1924, de Broglie published a paper titled “Research on the Theory of Quanta,” which introduced the concept of matter waves. His theory suggested that electrons, like light, coud exhibit both wave and particle behavior, depending on the experimental setup.
De Broglie’s theory was later confirmed by experiments conducted by Clinton Davisson and Lester Germer in 1927. They observed electron diffraction patterns, which were consistent with de Broglie’s theory of matter waves. This experiment provided strong evidence that electrons, previously thought of as purely particles, had a wave-like nature.
De Broglie’s work on matter waves was instrumental in the development of quantum mechanics, which revolutionized our understanding of the behavior of matter and energy on the atomic and subatomic level. His contributions to the field of physics earned him the Nobel Prize in Physics in 1929.
Demonstrating the Wave Nature of Electrons
The wave nature of electrons was first proposed by Louis de Broglie in 1924. However, it was not untl 1927 that the Davisson-Germer experiment provided direct experimental evidence for the wave nature of electrons.
Clinton Davisson and Lester Germer, two American physicists, were studying the diffraction of electrons off of crystalline surfaces. They observed a pattern of diffraction that could only be explained by the wave-like behavior of electrons. This experiment confirmed de Broglie’s hypothesis and provided important evidence for the wave-particle duality of matter.
The wave nature of electrons was first proposed by de Broglie, but it was the Davisson-Germer experiment that directly proved the wave-like behavior of electrons.
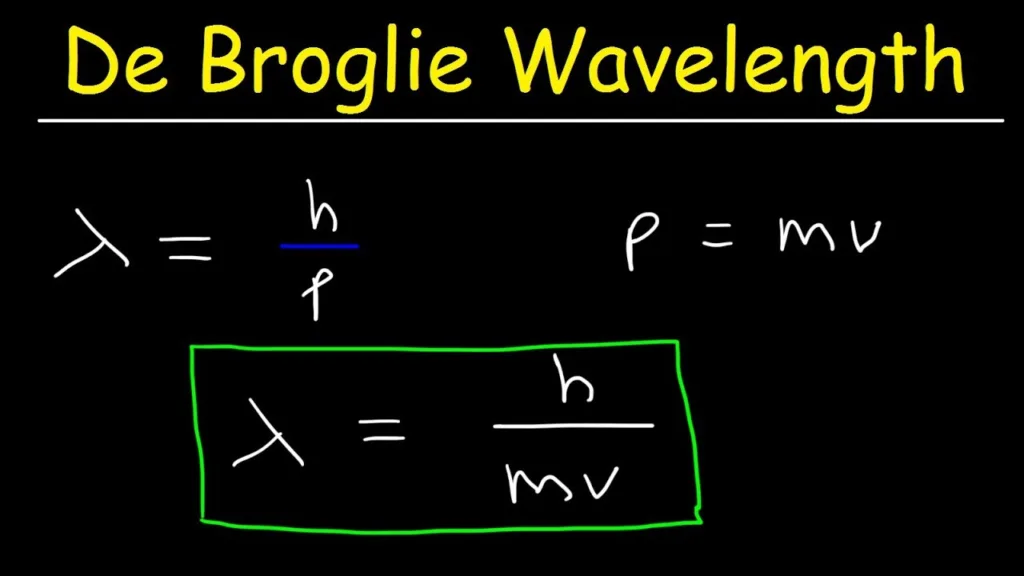
The Implications of the De Broglie Relationship
The De Broglie relationship states that all matter, including electrons, has both wave-like and particle-like properties. By measuring the wavelength of an electron, it is possible to determine the momentum of the electron. Conversely, by measuring the momentum of an electron, it is possible to determine its wavelength. This relationship can be used to understand the behavior of electrons in vrious systems, including atoms, molecules, and solids.
From the De Broglie relationship, it can be concluded that:
1. All matter, including electrons, has wave-like properties.
2. Electrons can be described as both particles and waves.
3. The wavelength of an electron is inversely proportional to its momentum.
4. The De Broglie relationship can be used to determine the momentum of an electron based on its wavelength.
5. The De Broglie relationship is important for understanding the behavior of electrons in various systems, including atoms, molecules, and solids.
Louis De Broglie’s Discovery of the Wave Nature of Electrons
Louis de Broglie discovered the wave nature of electrons in 1924. He proposed that particles, such as electrons, could be described not only as particles but also as waves. This was a revolutionary idea in the field of physics, as it challenged the classical notion of particles as discrete entities with well-defined positions and momenta.
De Broglie’s idea was initially met with skepticism, but it was later substantiated by experimental evidence. The wave-like behavior of electrons was observed in the way streams of electrons were reflected against crystals and spread through thin metal foils. This phenomenon, knwn as electron diffraction, provided strong evidence for de Broglie’s theory.
De Broglie’s discovery had far-reaching implications for our understanding of the nature of matter and the behavior of particles at the quantum level. It paved the way for the development of quantum mechanics and the modern understanding of subatomic particles.
Louis de Broglie discovered the wave nature of electrons by proposing that particles could be described as waves, which was later supported by experimental evidence of electron diffraction. This discovery revolutionized the field of physics and had significant implications for our understanding of the nature of matter.
Wave Theory of Electrons
The theory that describes electrons as waves is the Schrödinger model. This model is a fundamental concept in quantum mechanics and describes the behavior of electrons in terms of their wave-like properties. In the Schrödinger model, electrons are considered to be waves rather than particles, and their behavior is described by a mathematical function known as the wave function. This function gives the probability of finding an electron at a particular point in space. The Schrödinger model also introduces the concept of orbitals, which are regions of space where electrons are most likely to be found. These orbitals are characterized by their energy levels and shapes, and they play a crucial role in chemical bonding and the properties of materials. the Schrödinger model is an important tool for understanding the behavior of electrons and the structure of matter at the atomic and molecular levels.
To summarize, the Schrödinger model is a theory that describes electrons as waves and introduces the concept of orbitals to descrie their behavior. This model is a fundamental concept in quantum mechanics and plays a crucial role in understanding the properties of materials and chemical bonding.
Are Electrons Wave-Like?
Electrons are quantum objects that exhibit both wave-like and particle-like behavior. In certain experiments, they behave like particles, while in others, they behave like waves. This duality is a fundamental concept in quantum mechanics and can be explained by the wave-particle duality principle.
According to this principle, all quantum objects, including electrons, can be described by a wavefunction, which is a mathematical function that describes the probability of finding the particle at a particular location. This wavefunction behaves like a wave, meaning it can interfere with itself, diffract, and exhibit other wave-like properties.
However, when an electron is observed or measured, it behaves like a particle, meaning it takes on a specific position and momentum. This is known as the collapse of the wavefunction, were the probability wave collapses into a single point.
So, to answer the question, electrons are not literally waves, but they exhibit wave-like behavior and can be described by a wavefunction. They also exhibit particle-like behavior, depending on the experiment and observation.
The Wave-Like Nature of Electron
The electron is called a wave because it exhibits wave-like behavior in certain situations. When an electron is in motion, it creates a disturbance in the electromagnetic field that surrounds it. This disturbance can be thought of as a wave that propagates trough space.
The wave-like behavior of electrons was first discovered and explained by Louis de Broglie in 1924. He proposed that all matter, including electrons, has both particle-like and wave-like properties. This idea was supported by experiments that showed that electrons could diffract, or bend, like waves when they passed through narrow openings or around obstacles.
One of the key features of waves is that they have a wavelength, which is the distance between two successive peaks or troughs of the wave. Electrons also have a wavelength associated with them, which is inversely proportional to their momentum. This means that electrons with higher momentum have a shorter wavelength, while those with lower momentum have a longer wavelength.
The wave-like behavior of electrons has important consequences in many areas of physics and technology. For example, it is the basis for the operation of electron microscopes, which use the wave nature of electrons to achieve much higher resolution than optical microscopes. It is also important in the study of solid-state physics, where the behavior of electrons in materials is described using a wave-like model known as band theory.
Electrons are called waves because they exhibit wave-like behavior in certain situations, including diffraction and interference. This wave behavior is a fundamental aspect of the nature of matter, and has important implications in many areas of physics and technology.
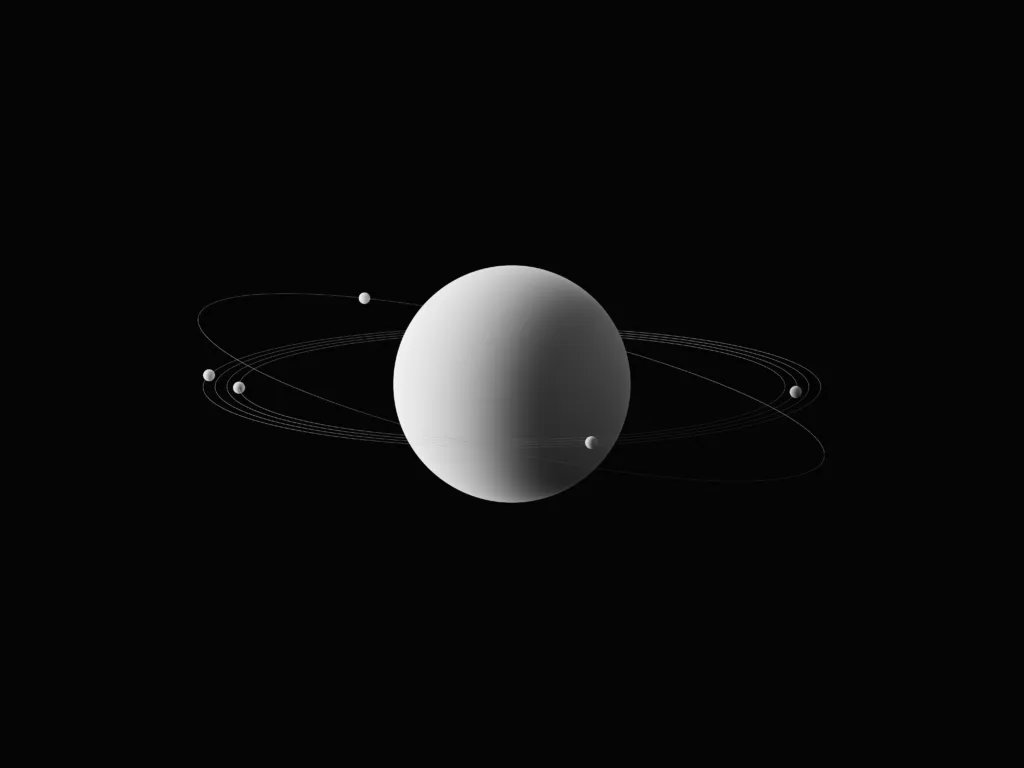
The Standing Wave Nature of Electrons
Electrons in atoms have quantized energies, which were discovered through experiments. This behavior was explained by imagining electrons as waves forming circular standing waves around the nucleus. A standing wave is a wave that appears to be standing still, and it is created when two waves of the same frequency and amplitude travel in opposite directions and interfere with each other.
To form a standing wave in a circle, an integral number of wavelengths must “fit” within the circumference. This is becase when a wave completes a full cycle and returns to its starting position, it must be in phase with itself to form a standing wave. If an integral number of wavelengths do not fit within the circumference, the wave will not be able to complete a full cycle and will not form a standing wave.
This concept is known as quantization, which means that only certain values are allowed for certain properties, such as the energy of an electron in an atom. These allowed values are called quantized energy levels, and they correspond to the standing waves that electrons can form around the nucleus. Each energy level corresponds to a specific standing wave, and electrons can only exist in these allowed energy levels.
Electrons are considered standing waves because their quantized energies can be explained by the formation of circular standing waves around the nucleus of an atom. This understanding has led to significant advancements in our understanding of atomic structure and the behavior of electrons in matter.
Conclusion
De Broglie’s suggestion that electrons have a wave nature revolutionized our understanding of the behavior of subatomic particles. He proposed that electrons should be considered as waves confined to the space around an atomic nucleus, and that these waves exist at specific frequencies. Through his work, De Broglie predicted that the mass of an electron was small enough to exhibit the properties of both particles and waves, and this was later confirmed experimentally.
The discovery of the wave properties of electrons has had a profound impact on our understanding of the behavior of matter at the atomic and subatomic level. It has led to the development of new technologies such as electron microscopy, and has contributed to our understanding of the structure and behavior of materials at the atomic level.
De Broglie’s work has had a lasting impact on our understanding of the fundamental nature of matter, and continues to influence scientific research and development to this day.
