Hexane is a hydrocarbon with the chemical formula C6H14. It is a highly volatile, flammable, and colorless liquid that is commonly used as a solvent in varios industrial applications. One of the most important characteristics of hexane is its polarity, i.e., whether it is polar or nonpolar. In this blog post, we will discuss in detail the polarity of hexane and the reasons behind it.
To understand the polarity of hexane, we need to first understand what polarity means. Polarity is a measure of the separation of electric charge in a molecule caused by the unequal sharing of electrons between atoms. In other words, it is a measure of how evenly or unevenly the electrons are distributed in a molecule. A molecule can be either polar or nonpolar, depending on the arrangement of its atoms and the distribution of electrons.
Now, coming to hexane, it is a nonpolar molecule. The reason behind this is the symmetric geometrical structure of hexane. Hexane has a linear structure, with six carbon (C) atoms and 14 hydrogen (H) atoms arranged in a straight line. Each carbon atom is bonded to two other carbon atoms and three hydrogen atoms. The electronegativity difference between carbon and hydrogen is very small, only 0.35, which means that the electrons are shared almost equally between them. Moreover, the carbon-carbon (C-C) bond is nonpolar because the electronegativity difference between two carbon atoms is zero. Therefore, the dipole moment of each C-H bond cancels out, resulting in a nonpolar molecule.
Another reason why hexane is nonpolar is the absence of any permanent dipole moment in its structure. Permanent dipole moment occurs when there is an unequal distribution of electrons in a molecule due to the presence of polar bonds or molecular geometry. As discussed earlier, hexane does not have any polar bonds, and its molecular geometry is symmetric, which results in the cancellation of any potential dipole moment.
It is important to note that the nonpolar nature of hexane makes it a good solvent for nonpolar substances. This is because, like dissolves like, and hexane can only dissolve other nonpolar substances. On the other hand, hexane is insoluble in polar solvents such as water.
Hexane is a nonpolar molecule due to its symmetric linear structure and the absence of any permanent dipole moment. This property makes it a useful solvent in certain industrial applications. Understanding the polarity of molecules is crucial in predicting their behavior in chemical reactions and their interactions with other substances.
The Non-Polar Nature of Hexane
Hexane is a hydrocarbon compound with the molecular formula C6H14. It is a colorless liquid with a characteristic odor and is commonly used as a solvent in various industrial processes. The polarity of a molecule is determined by the electronegativity difference between the atoms that make up the molecule.
In the case of hexane, the molecule is non-polar because the electronegativity difference between the hydrogen and carbon atoms is very small. The electronegativity is a measure of an atom’s ability to attract electrons towards itself in a covalent bond. Carbon and hydrogen atoms have similar electronegativities, with carbon being only slightly more electronegative than hydrogen. This means that the electrons in the covalent bonds between the carbon and hydrogen atoms are shared almost equally, resulting in a non-polar molecule.
Furthermore, hexane is composed of only carbon and hydrogen atoms, wich are both non-polar elements. The only intermolecular forces acting between hexane molecules are London dispersion forces, which are weak and temporary attractive forces that arise due to the fluctuations in the electron cloud around the molecule. These forces only exist when the molecules are in close proximity and do not result in a permanent dipole moment that would make the molecule polar.
Hexane is a non-polar molecule due to the small electronegativity difference between the carbon and hydrogen atoms, as well as the absence of any polar functional groups. The intermolecular forces between hexane molecules are weak London dispersion forces, which do not result in a permanent dipole moment.
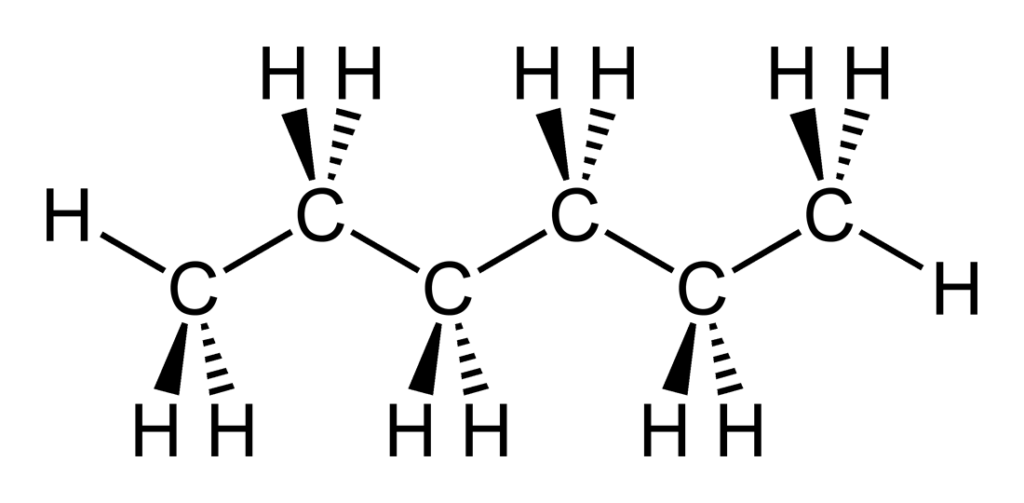
Is Hexane Polar or Nonpolar in Water?
Hexane is a nonpolar molecule that does not dissolve in water. Water is a polar molecule with a partially positive and partially negative end due to the unequal sharing of electrons between the hydrogen and oxygen atoms. In contrast, hexane is a hydrocarbon with only carbon and hydrogen atoms, and the electrons are shared equally between the atoms, resulting in a nonpolar molecule.
The polarity of a molecule determines its solubility in different solvents. Polar molecules dissolve in polar solvents, while nonpolar molecules dissolve in nonpolar solvents. Therefore, hexane will not dissolve in water because they are not compatible solvents.
It is important to note that some polar substances, such as alcohols, can dissolve in both polar and nonpolar solvents. However, this is due to the presence of a polar functional group in the molecule that can interact with both polar and nonpolar molecules.
Hexane is a nonpolar molecule that is not soluble in water. Polar and nonpolar substances have different solubilities and cnnot mix without the use of special techniques such as emulsifiers or surfactants.
Why Hexene Is Nonpolar
Hexene is a hydrocarbon molecule with the chemical formula C6H12. It is a type of alkene with a double bond between two of its carbon atoms. Hexene is used in the production of various chemical compounds and as a solvent in industrial processes.
The polarity of a molecule is determined by the distribution of its electrons and the shape of the molecule. In the case of hexene, the molecule has a symmetrical shape due to the presence of the double bond between two of its carbon atoms.
The carbon atoms in hexene are sp3 hybridized, whih means they have four electrons available for bonding. Each carbon atom in hexene is bonded to two other carbon atoms and two hydrogen atoms. The double bond between the two carbon atoms causes them to have a stronger attraction for the electrons in the bond, resulting in a slight negative charge on one end of the molecule and a slight positive charge on the other.
However, due to the symmetrical shape of the molecule, the positive and negative charges cancel each other out, resulting in a nonpolar molecule. This means that hexene is not soluble in polar solvents like water but is soluble in nonpolar solvents like hexane.
The nonpolarity of hexene is due to its symmetrical shape, which cancels out the slight charges on opposite ends of the molecule.
Solubility of Hexane in Water
N-hexane, also known as hexane, is a hydrocarbon with six carbon atoms and 14 hydrogen atoms, which makes it a non-polar molecule. Due to its non-polarity, it cannot form hydrogen bonds with water molecules, which are polar molecules. As a result, hexane is insoluble in water.
When we mix hexane and water, they form two separate layers due to their different polarities. The hexane layer will float on top of the water layer. This property is useful in varios industries, such as in the extraction of vegetable oils from plants, as hexane can dissolve the oils, leaving behind other unwanted substances.
It is essential to note that hexane can dissolve in some organic solvents, such as ethanol and acetone, which are also polar molecules. Hexane’s solubility in these solvents depends on the strength of their polarity and the size of the hexane molecule.
To summarize, hexane is not soluble in water due to its non-polarity. It forms two separate layers when mixed with water, with the hexane layer floating on top. However, it can dissolve in some organic solvents with polar properties.
The Insolubility of Hexane
Hexane is an organic compound with a molecular formula of C6H14. It is a hydrocarbon and belongs to the class of alkanes. Hexane is a non-polar molecule because it contins only carbon and hydrogen atoms, which have similar electronegativities, and thus, it has no net dipole moment.
On the other hand, water is a polar molecule because it contains a partially positive hydrogen end and a partially negative oxygen end. Due to this polarity, water molecules are attracted to each other through hydrogen bonds.
When hexane is introduced to water, the non-polar hexane molecules and polar water molecules cannot mix readily. Hexane molecules experience weak London dispersion forces with each other, but these forces are much weaker than the hydrogen bonds between water molecules. As a result, hexane cannot form hydrogen bonds with water and cannot dissolve in water.
In short, the insolubility of hexane in water is due to the non-polar nature of hexane compared to the polar nature of water. Hexane cannot form hydrogen bonds with water, leading to a lack of attraction between the two substances.

Why Alkanes Are Considered Non-Polar
Alkanes are referred to as nonpolar molecules because they are composed of carbon-carbon and carbon-hydrogen bonds that are nonpolar in nature. This means that the electrons in these bonds are evenly distributed, resulting in no significant charge differences across the molecule.
The electronegativity of carbon and hydrogen atoms is similar, which means that the electrons are shared equally between the atoms in the bond. This results in a symmetrical distribution of electrons around the molecule, with no areas of positive or negative charge.
Due to the nonpolar nature of alkanes, they are not soluble in water, which is a polar solvent. The lack of polarity also means that alkanes do not interact with other polar molecules, such as alcohols or amines.
Alkanes are generally less dense than water, which is why they tend to float on top of water when mixed together. This property makes them useful in varius applications, including as lubricants, fuels, and solvents.
The nonpolar nature of alkanes arises from the even distribution of electrons in the carbon-carbon and carbon-hydrogen bonds, resulting in no areas of significant charge differences across the molecule.
The Effects of Mixing Hexane and Water
When hexane is mixed with water, the two liquids do not mix and instead form two distinct layers. This is due to the differences in polarity between hexane and water molecules.
Water is a polar molecule, meaning it has a positive and negative end. The oxygen atom in water has a partial negative charge, while the hydrogen atoms have a partial positive charge. Hexane, on the oher hand, is a nonpolar molecule, meaning it has no positive or negative end. It is made up of only carbon and hydrogen atoms, which share electrons equally.
Because of these differences in polarity, hexane and water do not attract each other and do not mix. The hexane molecules will clump together and form a layer on top of the water, while the water molecules form a separate layer below.
It’s important to note that while hexane and water do not mix, they can still interact with each other. For example, some substances may dissolve in hexane but not in water, or vice versa. This property is often used in chemistry and industry to separate and purify different substances.
When hexane is mixed with water, the two liquids form two distinct layers due to the differences in polarity between the molecules.
Solubility of Hexane in Water
Hexane is a nonpolar hydrocarbon with the molecular formula C6H14. It is a colorless liquid that is commonly used as a solvent in various industrial and laboratory applications. On the other hand, water is a polar molecule with a bent structure, consisting of two hydrogen atoms and one oxygen atom. The oxygen atom has a partial negative charge, while the hydrogen atoms have a partial positive charge.
The solubility of a substance in water depends on the nature of the intermolecular forces between the solute and the solvent molecules. In the case of hexane and water, the intermolecular forces are different due to teir polarity. Hexane molecules are nonpolar and have weak London dispersion forces, which are attractive forces between nonpolar molecules. On the other hand, water molecules are polar and have strong hydrogen bonding between them.
Because of the difference in polarity, hexane and water do not mix well together. However, hexane is slightly soluble in water due to the small size of its molecules and the ability of water molecules to surround and disperse hexane molecules. The solubility of hexane in water is also enhanced by the presence of impurities, such as salts or other polar substances, which can form hydrogen bonds with water molecules and increase its polarity.
The solubility of hexane in water is limited due to the difference in polarity between the two substances. Hexane’s nonpolar nature and weak intermolecular forces make it less likely to dissolve in water, which is a polar substance with strong intermolecular forces.
Bonding Type of Hexane
Hexane, the chemical formula C6H14, is a molecule that is composed of carbon and hydrogen atoms bonded covalently. Covalent bonds are formed when two atoms share one or more pairs of electrons in order to achieve a stable electron configuration. In the case of hexane, each carbon atom is bonded to two oter carbon atoms and three hydrogen atoms, and each hydrogen atom is bonded to one carbon atom. This results in a linear, or straight-chain, structure for the molecule.
Covalent bonds are the most common type of chemical bond found in organic compounds, which are compounds that contain carbon atoms bonded to hydrogen atoms. Hexane is an organic compound because its skeletal structure is composed entirely of C-H bonds. The term “hydrocarbon” is also used to describe hexane, as it is a compound made up of only hydrogen and carbon atoms.
Hexane is a covalent molecule that is an organic compound and a hydrocarbon due to its composition of carbon and hydrogen atoms bonded together.
The Covalent Nature of Hexane
Hexane is a chemical compound that belongs to the group of alkanes. It has a molecular formula of C6H14, which means that it is made up of six carbon atoms and 14 hydrogen atoms. Since both carbon and hydrogen are non-metals, their bond formation in hexane is via covalent bonding.
Covalent bonding occurs when two atoms share their electrons to form a bond. In the case of hexane, carbon and hydrogen atoms share their electrons to form a covalent bond between them. This type of bond is very strong and stable, which makes hexane a stable compound.
Moreover, hexane is a non-polar molecule due to the equal sharing of electrons between carbon and hydrogen atoms. This means that the molecule has no charge separation and no positive or negative poles. Non-polar molecules have weak intermolecular forces between them, which makes hexane a liquid at room temperature and atmospheric pressure.
Hexane is a covalent compound becase it is made up of non-metals, carbon, and hydrogen that share their electrons to form a strong and stable bond. The equal sharing of electrons between the atoms also makes hexane a non-polar molecule with weak intermolecular forces.
Non-Polarity of Alkenes
Alkenes are nonpolar molecules due to their molecular structure. They consist of carbon-carbon and carbon-hydrogen bonds, which are considered nonpolar covalent bonds. In a nonpolar covalent bond, the electrons are shared equally between the two atoms, resulting in no net dipole moment. As a result, alkenes have no overall charge and do not have any poles, making them nonpolar.
This lack of polarity also means that alkenes are generally insoluble in water, as water is a polar molecule that tnds to dissolve other polar molecules. However, alkenes are soluble in nonpolar solvents like gasoline or oil.
It is important to note that the nonpolar nature of alkenes has implications for their chemical reactions. For example, alkenes tend to react with other nonpolar molecules, but not with polar molecules. This property makes alkenes useful starting materials for the synthesis of more complex organic molecules, as they can be selectively reacted with nonpolar reagents.
To summarize, the nonpolar nature of alkenes is due to the nonpolar covalent bonds between their carbon and hydrogen atoms, which results in no overall charge and no poles.
The Unique Properties of Hexane
Hexane is a colorless and flammable liquid with a distinct odor. It is a hydrocarbon, which means that it is composed of hydrogen and carbon atoms. What makes hexane special is its chemical properties, which make it useful in a variety of industrial and laboratory applications.
One of the most notable properties of hexane is its high flammability. This means that it can ignite easily and burn quickly, making it important to handle with care. Hexane is also volatile, which means that it can evaporate quickly into the air. When hexane evaporates, it can form explosive vapors, which can be dangerous if not handled properly.
In laboratories, hexane is used as a solvent for vaious purposes, such as extracting oils and fats, and cleaning glassware. It is a popular choice because it is relatively inexpensive and has a low toxicity compared to other solvents. However, it is important to use hexane in a well-ventilated area and with proper safety precautions.
In industry, hexane is often used in a mixture with other solvents to extract vegetable oils from crops such as soybeans. The solvent mixture is then separated from the oil and reused in the extraction process. This is an important process in the production of foods such as margarine, salad dressings, and mayonnaise.
Hexane is a special chemical due to its flammability and volatility, as well as its usefulness as a solvent in laboratory and industrial applications. However, it is important to handle hexane with care and follow proper safety procedures to avoid accidents and potential hazards.
The Benefits of Hexane as a Solvent
Hexane is considered one of the most effective solvents for oil extraction, and it is widely used for this purpose in the food industry. There are several reasons why hexane is the preferred solvent for oil extraction.
Firstly, hexane is a low toxicity solvent, meaning it is not harmful to humans or the environment when used in small amounts. This makes it a safer option compared to other solvents that may have adverse health effects.
Secondly, hexane has a low boiling point, whih makes it easy to remove from the extracted oil. This means that the final product will not have any residual solvent, ensuring a high-quality end product.
Thirdly, hexane is relatively inexpensive compared to other solvents, making it a cost-effective option for oil extraction.
In addition to oil extraction, hexane is also commonly used as a cleaning agent in various industries due to its ability to dissolve oils, greases, and other contaminants. It is widely used in the automotive, electronics, and manufacturing industries, among others.
Hexane is a highly effective solvent that offers several advantages for oil extraction and cleaning applications. However, it is important to use it safely and responsibly to minimize any potential health or environmental risks.

Solubility of Hexane in Salt Water
Hexane is a nonpolar organic compound with the molecular formula C6H14. It is a colorless liquid with a relatively low boiling point and is commonly used as a solvent in various industries. On the other hand, saltwater is a solution of salt (sodium chloride) in water. Sodium chloride is an ionic compound that dissociates in water, producing positively charged sodium ions (Na+) and negatively charged chloride ions (Cl-).
Due to the polar nature of water, it can dissolve ionic compounds like sodium chloride. However, hexane is a nonpolar solvent that cannot dissolve ionic compounds like salt. Therefore, hexane is not soluble in saltwater.
Hexane is not soluble in saltwater due to the difference in polarity btween the two substances. While salt dissolves in water due to its ionic nature, hexane cannot dissolve in saltwater due to its nonpolar nature.
The Hydrophobicity of Hexane
Hexane, specifically n-hexane, is a hydrophobic compound, meaning it is not soluble in water. This is due to its molecular structure and properties. Hexane is composed of carbon and hydrogen atoms, whih form nonpolar covalent bonds. Water, on the other hand, is a polar molecule, meaning it has a positive and negative end due to its asymmetric shape and the presence of oxygen. Polar molecules tend to dissolve in other polar substances, while nonpolar molecules tend to dissolve in nonpolar substances.
The hydrophobic nature of hexane can be quantified by its limited solubility in water, which is only 9.5 mg per liter at 25 °C and 1 atm. This is due to its high dimensionless Henry’s law constant of 73.7, which is a measure of its vapor-liquid partition coefficient. This means that hexane has a strong tendency to remain in the gas phase rather than dissolve in water.
In contrast, hydrophilic compounds are soluble in water due to their polar or charged nature. They can form hydrogen bonds with water molecules, which allows them to dissolve in water. Examples of hydrophilic compounds include salts, alcohols, and sugars.
To summarize, hexane is a hydrophobic compound that is not soluble in water due to its nonpolar molecular structure and properties. Its limited solubility in water can be quantified by its high Henry’s law constant.
Conclusion
Hexane is a nonpolar molecule due to its symmetric geometrical structure and the small electronegativity difference between carbon and hydrogen atoms. This nonpolarity makes hexane insoluble in water and a suitable solvent for nonpolar substances. The London dispersion forces hold hexane molecules together in a liquid state. Understanding the polarity of hexane is essential in various scientific fields, including chemistry, biology, and environmental science. hexane’s nonpolarity plays a crucial role in its physical and chemical properties, making it a valuable substance in many industrial applications.
