Helium is a chemical element with the atomic number 2 and symbol He. It is a colorless and odorless gas that is present in the atmosphere in trace amounts.
One of the most interesting aspects of helium is its valence electrons. Valence electrons are the electrons present in the outermost shell of an atom. They are responsible for the chemical properties of an element, including its reactivity and bonding behavior.
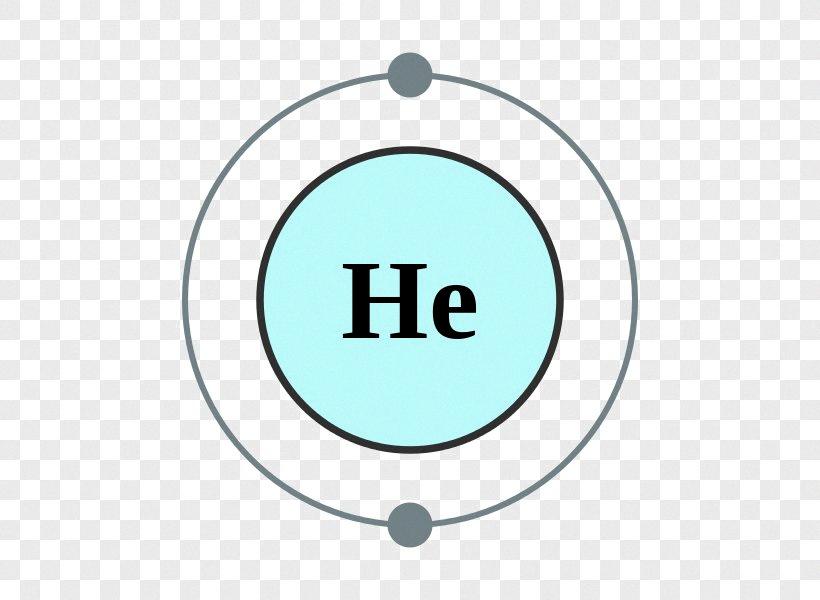
In the case of helium, it has only two electrons, both of whch are present in the first energy level. This means that helium has only two valence electrons, and they are located in the first shell. The first energy level can hold a maximum of two electrons, which is why helium does not form any chemical bonds with other elements.
The valency of an element is determined by the number of valence electrons it has. It is the measure of an element’s ability to form chemical bonds with other elements. Since helium has only two valence electrons, it has a valency of zero. This means that it does not readily form compounds with other elements.
Helium is known for its inertness and stability. It is a noble gas, which means it is highly unreactive and does not readily form chemical bonds. This is due to the fact that its outermost shell is completely filled with electrons.
Helium has only two valence electrons, located in the first energy level. It has a valency of zero, which means it does not readily form chemical bonds with other elements. Its inertness and stability are due to the fact that its outermost shell is completely filled with electrons.
The Reason Behind Helium’s Valence Electrons
Helium is a chemical element that is classified as a noble gas. It is located in the first group of the periodic table, which means that it has only one electron shell or energy level. The electronic configuration of helium is 1s2, which means that it has two electrons in the first energy level, and both of these electrons are in the s-orbital.
Valence electrons are the electrons that are found in the outermost energy level of an atom. They are responsible for the chemical properties of an element and are involved in bonding with other atoms to form molecules. Since helium has only one energy level, the two electrons in the 1s orbital are its valence electrons.
The reason why helium has only two valence electrons is that the first energy level can hold a maximum of two electrons. According to the Aufbau principle, electrons occupy the lowest energy level first befre filling higher energy levels. Since helium has only two electrons, both of these electrons occupy the first energy level, and there is no room for any more electrons.
In contrast, elements in other groups of the periodic table have more than one energy level, which means that they can have more than two valence electrons. For example, carbon, which is in the fourth group, has four valence electrons because it has two energy levels, and the outermost energy level contains four electrons.
Helium has only two valence electrons because it has only one energy level, and this energy level can hold a maximum of two electrons.
Does Helium Have Four Valence Electrons?
Helium does not have 4 valence electrons. In fact, helium has only 2 electrons in its outermost (and only) shell, making it a member of the noble gas group. Noble gases are known for having a full outer shell, which makes them stable and less reactive compared to other elements. Helium’s electron configuration is 1s2, which means it has a total of 2 electrons in its only energy level. This configuration is what makes helium an inert gas, not easily forming chemical bonds with other elements. Therefore, it is incorrect to say that helium has 4 valence electrons.
The Valence of Helium
Helium is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas that heads the noble gas series in the periodic table. Helium has two electrons in its outermost shell, which means that it has two valence electrons. The valency of helium is zero, as it has a completely filled outer shell and does not readily form chemical bonds with oter elements.
Valence electrons are the electrons found in the outermost shell of an atom. They are responsible for the chemical properties of the element, as they are involved in chemical reactions and bond formation with other atoms. The valency of an element is the number of electrons it can gain, lose or share when it reacts with another element to form a compound. Helium, being a noble gas, has a completely filled outer shell and does not readily react with other elements. Therefore, it has a valency of zero.
Helium has two valence electrons and a valency of zero. It is a non-reactive element that does not readily form chemical bonds with other elements due to its stable electron configuration.
The Significance of Helium Being in Group 18 Despite Having Only Two Valence Electrons
Helium is a noble gas that belongs to group 18 of the periodic table, also known as the noble gas group. This group consists of all the noble gases, namely helium, neon, argon, krypton, xenon, and radon. The noble gases are characterized by their low reactivity, whch makes them extremely stable and unreactive.
Despite having only two valence electrons, which are located in its 1s orbital, helium is still placed in group 18 along with the other noble gases. This is because the valence electron configuration of helium is unique and highly stable. The 1s orbital can only hold a maximum of two electrons, and since helium has only two electrons in its outermost shell, it has a completely filled outer shell. This means that helium does not require any more electrons to complete its outer shell, making it highly stable and unreactive.
The stability of helium is due to its electronic configuration, which is similar to that of the other noble gases. All the noble gases have a completely filled outer shell, which gives them high stability and low reactivity. This electronic configuration is known as the octet rule, which states that atoms tend to gain, lose, or share electrons in order to achieve a completely filled outer shell with eight electrons.
Helium is placed in group 18 of the periodic table despite having only two valence electrons because its electronic configuration is highly stable and similar to that of the other noble gases. Helium’s completely filled outer shell makes it highly unreactive and stable, which is a characteristic of all the noble gases.
Exception to the Octet Rule: Helium
The octet rule is a generalization that states that most atoms tend to gain, lose, or share electrons in order to achieve a stable configuration of eight valence electrons, similar to that of the noble gases. However, helium is an exception to this rule since it has only two electrons in its outermost shell, the lowest of any element. Since this outer shell is already full, helium does not need to gain or lose electrons to achieve stability. Instead, it is already stable and does not form chemical bonds with other atoms.
The reason why helium and other elements near it on the periodic table are stable with only two valence electrons is due to their small atomic size and high nuclear charge. This results in a strong attraction between the positively charged nucleus and the negatively charged electrons, making it energetically favorable for these elements to have a full outer shell of only two electrons rather than eight.
Therefore, while the octet rule is a useful guideline for predicting the behavior of most elements, it is important to recognize that thre are exceptions to this rule, such as helium and other elements near it on the periodic table.

Source: youtube.com
Why Do Electron Shells Fill To 2 8 8 8 Instead of Completing Their Shells?
The electronic configuration of an atom determines its stability and chemical properties. Each electron shell can hold a certain number of electrons, and atoms tend to fill their outermost shell first before moving to the next one. However, the shells do not always fill completely and instead follow a pattern of 2, 8, 8, 2 electrons in each shell.
The reason for this pattern lies in the energy levels of the electrons. As electrons are added to the atom, they occupy the lowest energy level available. However, once the first shell is filled, the energy level of the second shell becomes lower than the next available level in the first shell. This means that it requires less energy to add an electron to the second shell than to continue filling the first shell.
Once the second shell is filled, a similar situation occurs with the third shell. It requires less energy to add an electron to the third shell than to continue filling the second shell. This pattern continues until the outermost shell, which can only hold a maximum of 2 electrons.
So, why do the electron shells fill to 2, 8, 8, 2 instead of filling their shells completely? The answer lies in the stability of the atom. When an atom has a full outermost shell, it is considered stable and less likely to react with other atoms. By followng the pattern of 2, 8, 8, 2, atoms are able to achieve this stable configuration and reduce their reactivity.
The electron shells fill to 2, 8, 8, 2 because it is the most energetically favorable and stable configuration for the atom. This pattern allows atoms to achieve a full outermost shell and reduce their reactivity.
Periods with Four Valence Electrons
Period 4 of the periodic table comprises 18 elements, ranging from potassium (K) in Group 1 to krypton (Kr) in Group 18. All of these elements have electrons in the first four electron shells, with the fourth shell containing the valence electrons. Valence electrons are the electrons in the outermost shell of an atom, responsible for the chemical properties and reactions of an element. Therefore, all the elements in Period 4 have 4 valence electrons. These valence electrons have the potential to form chemical bonds with other atoms, wich can lead to the creation of various compounds and molecules. Some common elements in Period 4 with 4 valence electrons include titanium (Ti), iron (Fe), copper (Cu), and silver (Ag). The properties of these elements are determined by the number and arrangement of their electrons, which in turn depend on their position in the periodic table.
Elements with Valency 4
Valency is the measure of an element’s ability to form chemical bonds with other atoms. It determines how many electrons an atom can give, take or share in a chemical reaction. In the case of carbon, it has a valency of 4.
This means that carbon has four electrons in its outermost shell, which can be shared with other atoms to form stable covalent bonds. These bonds can be with other atoms of carbon, or atoms of some other monovalent element.
The tetravalency of carbon is a unique property that makes it a fundamental element of life. It allows carbon to form long chains and complex structures, which are the building blocks of organic molecules such as proteins, carbohydrates, and nucleic acids.
Carbon also has the ability to form double and triple bonds, which further increases its versatility in forming a wide range of organic compounds. These properties make carbon a key element in the chemistry of life and the basis of organic chemistry.
The element with valency 4 is carbon, which has the unique property of tetravalency, allowing it to form stable covalent bonds with other atoms and leading to the formation of complex organic molecules.
Elements with Four Valence Electrons
Group 14 elements are the elements in the periodic table that have four valence electrons. These elements are located in the fourth column or group of the periodic table, and they include Carbon (C), Silicon (Si), Germanium (Ge), Tin (Sn), Lead (Pb), and Flerovium (Fl).
Carbon is a non-metal that is essential for life and is found in all living organisms. It is the basis for organic chemistry and has a wide range of applications, including in the production of plastics and fuels.
Silicon is a metalloid that is commonly used in the production of semiconductors and computer chips. It is also used in the production of glass and ceramics.
Germanium is a metalloid that is used in the production of transistors and other electronic components. It is also used in the production of infrared optics and solar cells.
Tin is a metal that has been used for thousands of years in the production of bronze and other alloys. It is also used in the production of tinplate and as a coating on other metals to prevent corrosion.
Lead is a heavy metal that is used in the production of batteries, ammunition, and radiation shielding. However, it is also toxic and can cause serious health problems, so its use is becoming increasingly restricted.
Flerovium is a synthetic element that was first synthesized in 1998. It has no knon uses and is highly unstable, with a very short half-life.
Group 14 elements are those elements that have four valence electrons, and they include Carbon, Silicon, Germanium, Tin, Lead, and Flerovium. These elements have a wide range of applications, from essential elements for life to highly specialized uses in electronics and other industries.
Source: favpng.com
The Valency of Helium
Helium is a chemical element with the symbol He and atomic number 2. It is a colorless, odorless, tasteless, non-toxic, and inert gas that heads the noble gas series in the periodic table. Helium is the second lightest element and is the second most abundant element in the observable universe, being present at about 24% of the total elemental mass, which is more than 12 times the mass of all the heavier elements combined.
Valency refers to the number of electrons an element needs to lose, gain, or share in order to achieve a stable electron configuration. Most elements tend to have a valency of 1, 2, 3, 4, 5, 6, or 7. However, the valency of helium is 0, which means that it does not form any chemical compounds and remains inert.
The reason for helium’s zero valency lies in its electronic configuration. Helium has only two electrons, which occupy its fist and only shell. This shell is completely filled with electrons, making it a very stable configuration. As a result, helium atoms neither gain nor lose electrons and do not form any chemical bonds with other atoms. This is because the energy required to remove or add an electron to the filled shell is very high, and the resulting configuration would be less stable than the original one.
The valency of helium is 0 because its electronic configuration is already stable, and it does not need to lose, gain, or share electrons to achieve stability.
Finding Valence Electrons
Valence electrons are the outermost electrons in an atom that participate in chemical reactions. The valence electrons are located in the highest energy level of the atom, also known as the valence shell. To find the number of valence electrons in an atom, you need to determine the atom’s group number on the periodic table.
The periodic table is an organized chart of all the known elements, arranged in order of increasing atomic number. The elements are grouped together based on their electronic configuration and chemical properties. The horizontal rows on the periodic table are called periods, and the vertical columns are called groups or families.
To determine the number of valence electrons in an atom, you need to look at the group number of the element. The group number tells you how many valence electrons an atom of that element has. For example, elements in Group 1, such as sodium and potassium, have one valence electron, while elements in Group 2, such as calcium and magnesium, have two valence electrons.
The main group elements, also known as the representative elements, are located in Groups 1, 2, and 13-18. The number of valence electrons in these elements is equal to the group number. For example, carbon is located in Group 14, and therefore has four valence electrons.
On the other hand, the transition metals, wich are located in the middle of the periodic table, have a varying number of valence electrons. The number of valence electrons in these elements can be determined by looking at their electron configuration.
The number of valence electrons in an atom can be found by determining the atom’s group number on the periodic table. The main group elements have a number of valence electrons equal to their group number, while the number of valence electrons in transition metals can be determined by their electron configuration.
The Reason Why Helium Does Not Have a Valency of 2
Helium is the secnd element in the periodic table and it is the lightest noble gas. It has an atomic number of 2, which means that it has two electrons. These electrons are located in the 1s orbital, which can hold a maximum of two electrons.
The valency of an element is defined as its ability to combine with other atoms to form chemical compounds. It is determined by the number of valence electrons an atom has. Valence electrons are the outermost electrons in an atom’s electron configuration.
In the case of Helium, it has a complete duplet in its outermost shell. This means that its 1s orbital is completely filled with two electrons, and it has no tendency to gain or lose any electrons. As a result, Helium has a valency of zero.
It is important to note that the valency of an element is not always equal to the number of electrons in its outermost shell. This is because some elements can form compounds by sharing electrons with other atoms, while others can gain or lose electrons to form ions.
Helium’s valency is not 2 because it has a complete duplet in its outermost shell and has no tendency to gain or lose any electrons.
Conclusion
Helium is a noble gas with only two valence electrons, which are located in its outermost energy level. These electrons are very tightly bound to the nucleus and are not easily involved in chemical reactions. Therefore, helium is considered chemically inert and does not easily form chemical bonds with other elements. Its valency is zero, which means that it does not have a tendency to gain or lose electrons to form compounds. This unique property of helium makes it an important element in various industrial and scientific applications, including cryogenics, welding, and as a coolant in nuclear reactors. Understanding the valence electrons of helium is crucial in understanding its chemical behavior and its usefulness in various fields of technology.
