When studying chemistry, you will oten come across the terms “grams” and “atoms”. These two units of measurement are essential in understanding the composition of matter at a molecular level. In this article, we will discuss how to convert grams to atoms and vice versa.
Avogadro’s number is the key to understanding the relationship between grams and atoms. It is defined as the number of atoms or molecules in one mole of a substance, which is 6.022 × 10^23. This number is crucial in chemistry because it allows us to convert between grams and atoms.
To convert from grams to atoms, we need to know the atomic weight of the element. The atomic weight is the average mass of one atom of an element, expressed in atomic mass units (amu). For instance, the atomic weight of carbon is 12.011 amu. To convert 10 grams of carbon to atoms, we divide the mass by the atomic weight and then multiply by Avogadro’s number:
10 g / 12.011 amu = 0.832 mol
0.832 mol x 6.022 × 10^23 atoms/mol = 5.01 × 10^23 atoms
Therefore, 10 grams of carbon contain approximately 5.01 × 10^23 atoms. You can use this same method to convert grams to atoms for any element, as long as you know the atomic weight.
Conversely, we can also convert from atoms to grams by using Avogadro’s number and the atomic weight. To do this, we divide the number of atoms by Avogadro’s number and then multiply by the atomic weight:
5.01 × 10^23 atoms / 6.022 × 10^23 atoms/mol = 0.833 mol
0.833 mol x 12.011 amu = 10.00 g
Therefore, 5.01 × 10^23 atoms of carbon weigh approximately 10 grams.
It is essential to note that the conversion factor between grams and atoms depends on the specific element’s atomic weight. Therefore, it is crucial to have a periodic table of elements on hand when working with grams and atoms.
Converting between grams and atoms is a fundamental concept in chemistry that requires knowledge of Avogadro’s number and the atomic weight of the element. By using these two values, we can easily convert between grams and atoms for any element.
Number of Atoms in a Gram
The number of atoms in a gram depends on the atomic weight of the element in question. Avogadro’s number, which is 6.022 × 1023 atoms/mole, is used to calculate the number of atoms in a given sample of an element.
To determine the number of atoms in a gram of an element, one must first calculate the number of moles in a gram of that element. This can be done using the atomic weight of the element, which is defined as the average mass of all the isotopes of that element.
For example, the atomic weight of hydrogen is 1 gram per mole. Therefore, one gram of hydrogen cotains one mole of hydrogen atoms, which in turn contains 6.022 × 1023 hydrogen atoms.
On the other hand, the atomic weight of carbon is 12 grams per mole. Therefore, one gram of carbon contains 1/12 moles of carbon atoms, which equals to 5.018 × 1022 carbon atoms.
The number of atoms in a gram depends on the atomic weight of the element. Using Avogadro’s number, one can calculate the number of atoms in a given sample of an element by first determining the number of moles in that sample.
Source: general.chemistrysteps.com
Finding the Number of Grams in an Atom
To find how many grams are in an atom, you need to know the relative atomic mass of the element. The relative atomic mass is the average mass of an atom of that element, taking into account the different isotopes and their abundance. This vale is usually given in the periodic table.
Once you have the relative atomic mass, you can convert it to grams using Avogadro’s number. Avogadro’s number is the number of atoms in one mole of the element, and it is approximately 6.022 x 10^23.
To convert the relative atomic mass to grams, you simply divide it by Avogadro’s number. For example, the relative atomic mass of carbon is 12.011, so the mass of one carbon atom is:
12.011 / 6.022 x 10^23 = 1.99 x 10^-23 grams
This means that one carbon atom has a mass of approximately 2 x 10^-23 grams.
If you want to find the mass of a larger number of atoms, you can use the same formula but multiply the result by the number of atoms. For example, if you have 10^24 carbon atoms, the mass would be:
(12.011 / 6.022 x 10^23) x 10^24 = 1.99 x 10^1 grams
This means that 10^24 carbon atoms have a mass of approximately 20 grams.
In summary, to find how many grams are in an atom, you need to know the relative atomic mass of the element and divide it by Avogadro’s number.
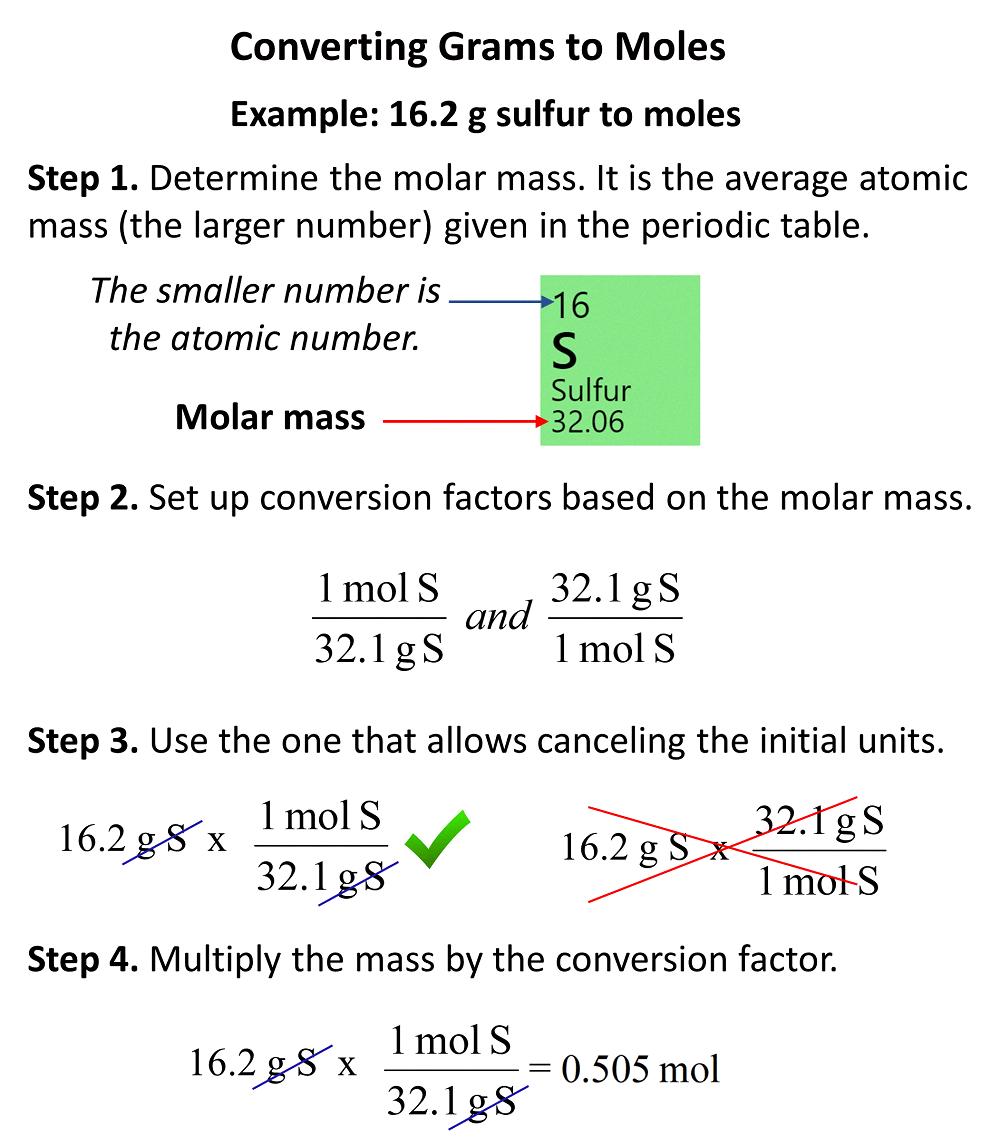
Converting Grams to Moles of Atoms
The number of moles of atoms in grams depends on the atomic mass of the element. To calculate the number of moles of atoms in a gien mass, we need to use the formula:
Moles = mass ÷ atomic mass
For example, if we have 10 grams of carbon-12, we can calculate the number of moles of atoms using its atomic mass, which is 12.01 g/mol. So,
Moles = 10 g ÷ 12.01 g/mol = 0.833 moles
This means that there are 0.833 moles of carbon-12 atoms in 10 grams of carbon-12.
Similarly, if we have 20 grams of oxygen-16, we can calculate the number of moles of atoms using its atomic mass, which is 15.99 g/mol. So,
Moles = 20 g ÷ 15.99 g/mol = 1.251 moles
This means that there are 1.251 moles of oxygen-16 atoms in 20 grams of oxygen-16.
In general, the number of moles of atoms in a given mass depends on the atomic mass of the element and the mass of the sample. We can use the formula mentioned above to calculate the number of moles of atoms in any given mass of an element.
Number of Atoms in 1 Gram of Oxygen
Oxygen is an essential element that plays a vital role in the composition of the earth’s atmosphere. It is a diatomic molecule, whih means that two oxygen atoms combine to form one molecule of oxygen. The molecular formula for oxygen is O2, and its atomic weight is 16 grams.
The number of atoms in 1g of oxygen can be calculated by using Avogadro’s number, which is 6.022 × 10^23. This number represents the number of particles in one mole of a substance. In the case of oxygen, one mole of oxygen contains 6.022 × 10^23 oxygen atoms.
Therefore, to determine the number of atoms in 1g of oxygen, we need to find the number of moles of oxygen in 1g. This can be calculated by dividing the given mass (1g) by the atomic weight of oxygen (16g).
1g/16g = 0.0625 moles of oxygen
Multiplying this value by Avogadro’s number gives us the number of atoms in 1g of oxygen:
0.0625 moles × 6.022 × 10^23 atoms/mole = 3.76 × 10^22 atoms
Hence, there are approximately 3.76 × 10^22 oxygen atoms in 1g of oxygen.
Number of Atoms in 1 Gram of Water
Water is a molecule composed of two hydrogen atoms (H) and one oxygen atom (O), with the chemical formula H2O. To determine the number of atoms in 1g of water, we need to use the Avogadro’s number, which is approximately 6.022 × 10^23.
First, we need to calculate the molar mass of water, which is the mass of one mole of water molecules. The molar mass of water is:
2(1.008 g/mol) + 1(15.999 g/mol) = 18.015 g/mol
This means that one mole of water molecules weighs 18.015 g, and conains 6.022 × 10^23 water molecules.
To determine the number of atoms in 1g of water, we need to convert 1g to moles. We can do this by dividing 1g by the molar mass of water:
1g ÷ 18.015 g/mol = 0.0555 mol
Therefore, 1g of water contains 0.0555 moles of water molecules. To determine the number of atoms in 1g of water, we need to multiply the number of water molecules by the number of atoms in each molecule:
0.0555 mol × 6.022 × 10^23 atoms/mol = 3.34 × 10^22 atoms
So, there are approximately 3.34 × 10^22 atoms in 1g of water.

Are Atoms Measured in Grams?
Atoms are extremely tiny particles that make up all matter in the universe. They have a mass, which is usually measured in atomic mass units (amu). However, this mass is not typically expressed in grams.
Atomic mass is a relative measure of the mass of an atom compared to the mass of a standard atom, which is usually carbon-12. The atomic mass takes into account the number of protons and neutrons in an atom’s nucleus. Protons and neutrons have a mass of approximately 1 amu each, while electrons have a negligible mass.
Therefore, the atomic mass of an element is the sum of the number of protons and neutrons in the nucleus. For example, the atomic mass of iron is 55.847 amu, which means that one iron atom has a mass of 55.847 atomic mass units.
While atomic mass is not expressed in grams, it is related to the concept of mole in chemistry. One mole is a unit of measurement that represents 6.02 x 10^23 particles of a substance. The atomic mass of an element, measured in amu, is the same as the mass in grams of one mole of that element. For example, one mole of iron atoms woud weigh 55.847 grams.
While atoms do have a mass, it is not typically expressed in grams. Instead, atomic mass is measured in atomic mass units (amu), which is a relative measure of the mass of an atom compared to the mass of a standard atom. However, atomic mass is related to the concept of mole in chemistry, and the atomic mass of an element in amu is the same as the mass in grams of one mole of that element.
Is One Gram Atom Equal to a Gram Atomic?
The terms “gram atomic mass” and “gram atom” are often used interchangeably, and they refer to the atomic mass expressed in grams. This means that one gram atom is equal to one gram atomic mass, which is the mass of a single atom expressed in grams. It is important to note that the gram atomic mass or gram atom is a unit of measurement that is commonly used in chemistry to express the mass of an atom or a group of atoms.
Furthermore, it is worth mentioning that the gram atomic mass or gram atom is not the same as the mole, which is anoter unit of measurement used to express the amount of a substance. The mole is defined as the amount of a substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are in 12 grams of carbon-12.
To summarize, gram atomic mass and gram atom are two terms that refer to the same thing, namely the atomic mass expressed in grams. Therefore, one gram atom is equal to one gram atomic mass.
Converting Grams to Moles
Converting grams to moles is a fundamental calculation in chemistry. It involves determining the number of molecules or atoms in a given amount of substance. This process is important because many chemical reactions require a specific amount of reactants to take place, and knowing the number of moles involved is essential for accurate calculations.
To convert grams to moles, you need to use the grams to moles formula, whih is:
N = m / M
Where:
N = number of moles
M = mass of substance in grams
M = molar mass of substance in grams per mole
To use this formula, you simply divide the mass of the substance in grams by its molar mass. The molar mass is the mass of one mole of the substance and is calculated by adding up the atomic masses of all the atoms in the molecule.
For example, let’s say you have 10 grams of sodium chloride (NaCl) and you want to convert it to moles. The molar mass of NaCl is 58.44 g/mol. Using the formula above, we get:
N = 10 g / 58.44 g/mol
N = 0.171 moles
Therefore, 10 grams of NaCl is equal to 0.171 moles of NaCl.
It’s important to note that the conversion from grams to moles depends on the molar mass of the substance. Therefore, it’s crucial to know the molar mass of the substance you’re working with. You can find this information on the periodic table or by using online resources.
To summarize, converting grams to moles is a simple process that involves using the grams to moles formula and the molar mass of the substance. By knowing the number of moles, you can accurately calculate the amount of substance needed for chemical reactions.
Calculating Atoms
Calculating the number of atoms is a fundamental concept in chemistry. It involves understanding the concept of moles and Avogadro’s number. Here’s how to calculate the number of atoms:
1. Determine the mass of the element/compound: The first step is to determine the mass of the element or compound in question. The mass can be given in grams or any other unit of mass.
2. Find the molar mass of the element/compound: The molar mass is the mass of one mole of the element or compound. It is expressed in grams per mole (g/mol). You can find the molar mass by adding the atomic masses of all the elements present in the compound. The atomic masses can be found on the periodic table.
3. Calculate the number of moles: Divide the mass of the element or compound by its molar mass to get the number of moles. The formula is:
Number of moles = Mass of element/compound ÷ Molar mass
4. Calculate the number of atoms: In one mole of any substance, there are 6.023 x 10^23 particles, which is known as Avogadro’s number. Therefore, to calculate the number of atoms, multiply the number of moles by Avogadro’s number. The formula is:
Number of atoms = Number of moles x Avogadro’s number
Here’s an eample to illustrate the calculation:
Example: Calculate the number of atoms in 2 grams of hydrogen gas (H2).
1. Determine the mass of hydrogen gas: The mass is 2 grams.
2. Find the molar mass of hydrogen gas: The molar mass of H2 is 2 grams per mole (g/mol).
3. Calculate the number of moles: Number of moles = 2 ÷ 2 = 1 mole.
4. Calculate the number of atoms: Number of atoms = 1 x 6.023 x 10^23 = 6.023 x 10^23 atoms.
Therefore, there are 6.023 x 10^23 atoms in 2 grams of hydrogen gas.
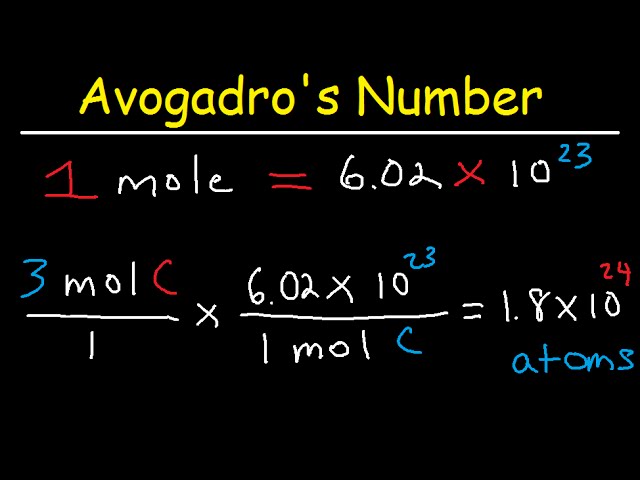
What Is the Weight of a Mole?
A mole is not exactly 1 gram, but it is very close. The mole is a unit used in chemistry to measure the amount of a substance. It is defined as the amount of a substance that contains the same number of particles (atoms, molecules, ions, etc.) as thre are in 12 grams of carbon-12. This number is known as Avogadro’s number, which is approximately 6.022 x 10^23 particles per mole.
The mass of 1 mole of a substance depends on its atomic or molecular weight. For example, the atomic weight of hydrogen is approximately 1 u (atomic mass unit), which means that 1 mole of hydrogen atoms (6.022 x 10^23 atoms) has a mass of approximately 1 gram. Similarly, the molecular weight of water is approximately 18 u, which means that 1 mole of water molecules (6.022 x 10^23 molecules) has a mass of approximately 18 grams.
It is important to note that the mass of 1 mole of a substance in grams is not always equal to its atomic or molecular weight in u. This is because the atomic mass unit is defined as 1/12 of the mass of a carbon-12 atom, which is not exactly 1 gram. However, the difference is very small, and for most practical purposes, the mass of 1 mole of a substance is considered to be equal to its atomic or molecular weight in grams.
To summarize, a mole is not exactly 1 gram, but the mass of 1 mole of a substance is very close to its atomic or molecular weight in grams. The exact mass of 1 mole of a substance in grams depends on its atomic or molecular weight, which is measured in atomic mass units (u).
Moles of Substance in 1 Gram
The number of moles in 1 gram can be calculated usig the molar mass constant, which is equal to 1 gram per mole. This constant is derived from the definition of the mole, which is the amount of a substance that contains the same number of entities (atoms, molecules, ions, etc.) as there are atoms in 12 grams of carbon-12.
To calculate the number of moles in 1 gram of a substance, we need to know its molar mass. The molar mass of a substance is the mass of one mole of its entities, expressed in grams per mole. It is calculated by summing the atomic masses of all the atoms in the substance’s chemical formula.
For example, the molar mass of water (H2O) is 18.02 grams per mole, which is the sum of the atomic masses of two hydrogen atoms (1.01 grams per mole each) and one oxygen atom (16.00 grams per mole).
Using this molar mass, we can calculate the number of moles in 1 gram of water as follows:
1 gram of water ÷ 18.02 grams per mole = 0.0555 moles of water
Therefore, 1 gram of water contains approximately 0.0555 moles of water.
The number of moles in 1 gram of a substance can be calculated by dividing 1 gram by the substance’s molar mass, which is expressed in grams per mole.
Converting 1 Mole to Grams
Converting moles to grams is a common calculation in chemistry. A mole is a unit used to measure the number of particles, such as atoms, molecules or ions, in a substance. The mass of one mole of a substance is called its molar mass and is expressed in grams per mole (g/mol).
To convert 1 mole to grams, you need to know the molar mass of the substance. The molar mass can be found by adding up the atomic masses of all the atoms in the molecule. For example, the molar mass of water (H2O) is 18.015 g/mol, which is calculated by adding the atomic masses of two hydrogen atoms (1.008 g/mol each) and one oxygen atom (15.999 g/mol).
Once you know the molar mass of the substance, you can convert 1 mole to grams by multiplying the molar mass by 1 mole. For example, if you want to convert 1 mole of water to grams, you wuld multiply the molar mass of water (18.015 g/mol) by 1 mole, which gives you 18.015 grams of water.
In summary, to convert 1 mole to grams, you need to:
1. Determine the molar mass of the substance
2. Multiply the molar mass by 1 mole
3. The result will be the mass of 1 mole of the substance in grams.
Conclusion
Avogadro’s number is an essential concept in chemistry that relates the number of atoms or molecules to the amount of a substance in grams. By dividing the relative atomic mass or molecular mass by Avogadro’s number, we can determine the number of grams per mole of the substance. Similarly, by adding up the atomic masses in a chemical formula and dividing by Avogadro’s number, we can find the mass of one molecule. Understanding the relationship beteen grams and atoms is crucial in various fields, including medicine, industry, and research. Therefore, it is crucial to comprehend the significance of Avogadro’s number and its application in chemistry.
