Liquid and gas liquid chromatography are powerful analytical techniques used to separate and analyze mixtures of chemical compounds. In both techniques, a stationary phase and a mobile phase are used to separate the compounds based on their physicochemical properties such as polarity, size, and solubility.
When it comes to liquid chromatography, the elution order of the compounds depends on the properties of the stationary phase and the mobile phase. For instance, in normal-phase liquid chromatography, the stationary phase is a polar material such as silica gel, alumina, or cellulose, and the mobile phase is a nonpolar solvent such as hexane, heptane, or ether. In this case, the more nonpolar compounds elute first, followed by the more polar ones.
On the other hand, in reverse-phase liquid chromatography, the stationary phase is a nonpolar material such as octadecylsilyl (ODS) or C18, and the mobile phase is a polar solvent such as water, methanol, or acetonitrile. In this case, the more polar compounds elute first, followed by the less polar ones.
Gas liquid chromatography, also knon as gas chromatography, works on the same principles as liquid chromatography but uses a gaseous mobile phase instead of a liquid one. In gas chromatography, the elution order of the compounds depends on their boiling points, with the lower boiling compounds eluting first.
It is worth noting that the elution order of the compounds can be influenced by several factors such as the column temperature, the flow rate of the mobile phase, the sample concentration, and the sample size. Therefore, it is important to optimize these parameters to achieve the best separation and resolution of the compounds.
The elution order of the compounds in liquid and gas liquid chromatography depends on the properties of the stationary and mobile phases, as well as other experimental factors. By understanding these factors, scientists can optimize their chromatographic separations and obtain accurate and reliable results.
Order of Elution in Gas Chromatography
Gas chromatography is a widely used technique in analytical chemistry to separate and analyze different components of a mixture. The order of elution in gas chromatography refers to the sequence in which the different components of a sample are separated and detected.
The order of elution in gas chromatography is primarily determined by the boiling points of the solutes. The stationary phase used in gas chromatography is typically a nonpolar liquid or polymer such as polydimethyl siloxane. Lower boiling solutes, which are more volatile and less polar, tend to elute first, followed by higher boiling solutes.
It is important to note that the order of elution can be influenced by factors such as the polarity of the stationary phase and the intermolecular interactions between the solutes and the stationary phase. For instance, replacing some of the methyl groups in polydimethyl siloxane with othr substituents can increase the stationary phase’s polarity and provide greater selectivity.
The order of elution in gas chromatography is primarily determined by the boiling points of the solutes, with lower boiling solutes eluting first. However, other factors such as the polarity of the stationary phase can also influence the order of elution.
Source: technologynetworks.com
What Compounds are Eluted First in Liquid Chromatography?
In liquid chromatography, the elution order of analytes depends on their polarity. Least polar analytes elute first, followed by more polar analytes that are retained longer.
To understand this concept, it’s important to know that liquid chromatography is a separation technique in which a mixture of analytes is passed through a stationary phase (usually a column packed with particles) and a mobile phase (a liquid that flows through the column). The analytes interact with the stationary phase differently depending on their polarity, which affects their retention time (the time it takes for an analyte to pass through the column).
The stationary phase in liquid chromatography is usually a polar or nonpolar material, such as silica or C18 (an alkyl-bonded silica material). Nonpolar analytes, such as hydrocarbons, have little interaction with the polar stationary phase and therefore elute first. Polar analytes, such as alcohols or carboxylic acids, have stronger interactions with the stationary phase and are retained longer.
Here is a summary of the elution order of analytes in liquid chromatography, from least polar to most polar:
– Nonpolar analytes (e.g. hydrocarbons)
– Aromatic compounds
– Alcohols and ethers
– Amines
– Carboxylic acids
– Strongly polar compounds (e.g. sugars, amino acids)
It’s important to note that the elution order can also be affected by oter factors, such as the mobile phase composition, pH, and temperature. However, the polarity of the analytes and the stationary phase is the primary factor that determines the elution order in liquid chromatography.
Factors Influencing Elution Order in Liquid Chromatography
Liquid chromatography is a widely used separation technique that involves the separation of solutes based on their interactions with a stationary phase and a mobile phase. In this technique, the elution order of solutes is determined by their polarity.
The stationary phase in liquid chromatography can be either polar or nonpolar. In a normal-phase separation, the stationary phase is polar, wile the mobile phase is nonpolar. The solutes that are less polar spend less time in the polar stationary phase and are the first solutes to elute from the column. This means that the elution order of solutes in normal-phase liquid chromatography is in the order of increasing polarity.
On the other hand, in a reversed-phase separation, the stationary phase is nonpolar, while the mobile phase is polar. In this case, the solutes that are more polar spend more time in the stationary phase and are the first solutes to elute from the column. This means that the elution order of solutes in reversed-phase liquid chromatography is in the order of decreasing polarity.
Other factors that can influence the elution order of solutes in liquid chromatography include the size, shape, and charge of the solutes, as well as the pH and temperature of the mobile phase. However, polarity remains the primary factor that determines the elution order of solutes in liquid chromatography.
Does Nonpolar Molecule Elution Occur First in Gas Chromatography?
Gas chromatography is a technique used to separate and analyze different compounds in a mixture. The process involves passing a gaseous mixture of compounds through a long column packed with a stationary phase material. The stationary phase material is typically a solid material coated with a liquid film, which interacts with the compounds in the mixture differently based on their polarity.
In gas chromatography, the elution order of compounds is determined by their polarity. Nonpolar compounds, which have weaker interactions with the stationary phase, will elute first in the process. This is because nonpolar compounds are less attracted to the stationary phase and more easily carried by the mobile phase, which is typically an inert gas like helium or nitrogen.
Polar compounds, on the other hand, have stronger interactions with the stationary phase and will elute later in the process. Polar compounds are more attracted to the stationary phase and are less easily carried by the mobile phase. As a result, they spend more time interacting with the stationary phase and take longer to elute from the column.
To summarize, the elution order in gas chromatography is determined by the polarity of the compounds in the mixture. Nonpolar compounds will elute first, followd by polar compounds.
Which Substance Will Elute First?
In general column chromatography, the elution process is dependent on the interactions between the compounds and the stationary and mobile phases. The elution order of compounds is manly determined by their polarity. Non-polar compounds have a weak interaction with the stationary phase and a stronger interaction with the mobile phase, so they tend to elute first. On the other hand, polar compounds have a strong interaction with the stationary phase and a weaker interaction with the mobile phase, so they tend to elute last. However, there are exceptions to this rule, and the elution order can also be influenced by other factors such as the size, shape, and charge of the molecules.
To summarize, in column chromatography, non-polar compounds will typically elute first, followed by moderately polar compounds, and finally polar compounds. It is important to note that this is a general rule and may not always hold true in all cases.
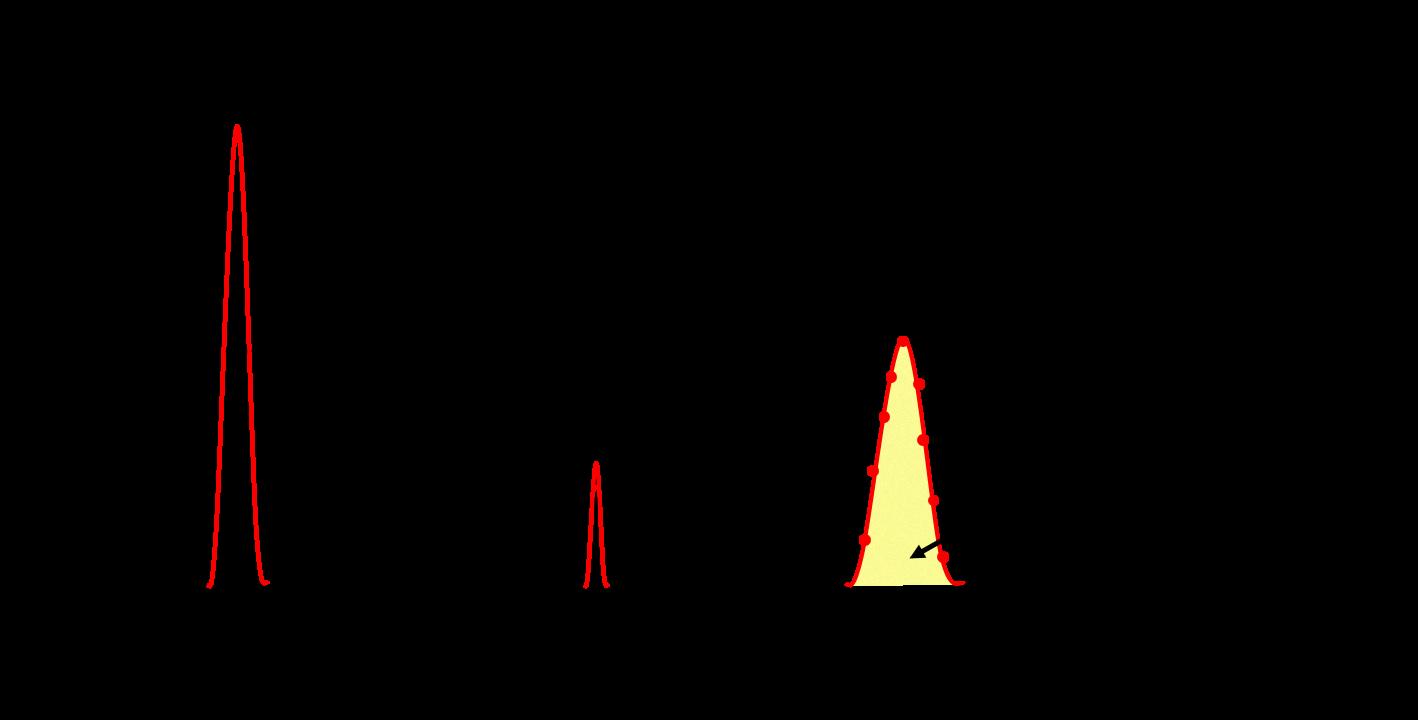
Order of Elution in Gas Chromatography
Gas chromatography is a widely used technique for separating and analyzing different components of a mixture. The elution order of components in this technique depends on their boiling points and their affinity for the stationary phase. The thumb rule in gas chromatography is that the component with the lowest boiling point will elute first from the column.
Therefore, in a gas chromatography column, the component with the lowest boiling point will be the first to elute. This is because the component with the lowest boiling point will have the highest vapor pressure, and hence, it will be the most volatile. As the sample is introduced into the column, the components will interact with the stationary phase and the mobile phase, resulting in their separation based on their boiling points.
For example, if we have a mixture of methanol, ethanol, and propanol, the component with the lowest boiling point, methanol, will elute first, followed by ethanol and then propanol. This is because methanol has the lowest boiling point among the three and hnce is the most volatile.
The elution order of components in gas chromatography is based on their boiling points and their affinity for the stationary phase. The component with the lowest boiling point will elute first from the column.
Benefits of Non-Polar Elutes Being First in Chromatography
Non-polar compounds elute firt in chromatography because the stationary phase used in the column is polar. Polar stationary phases have polar functional groups, such as hydroxyl or amino groups, which can form hydrogen bonds with polar molecules. This interaction between the polar stationary phase and polar molecules results in a stronger retention of polar compounds in the column, while non-polar compounds are not retained and elute first.
In contrast, reversed-phase chromatography uses a non-polar stationary phase, such as a hydrocarbon chain, that retains non-polar molecules, leaving polar molecules to elute first. Non-polar molecules are attracted to the non-polar stationary phase through Van der Waals interactions, while polar molecules are repelled and elute quickly.
The order of elution in chromatography is also determined by the polarity of the mobile phase used. If a polar mobile phase is used with a polar stationary phase, polar molecules will elute later than non-polar molecules. Conversely, if a non-polar mobile phase is used with a non-polar stationary phase, non-polar molecules will elute later than polar molecules.
The order of elution in chromatography is determined by the polarity of both the stationary phase and the mobile phase. In polar stationary phase chromatography, non-polar compounds elute first because they are not retained by the polar stationary phase, while in reversed-phase chromatography, polar molecules elute first because they are not retained by the non-polar stationary phase.
The Impact of Molecular Size on Elution
Size exclusion chromatography is a technique that separates molecules based on their size. The stationary phase in this technique is a porous resin, which acts as a molecular sieve. The pores in the resin allow smaller molecules to penetrate and travel through the column, while larger molecules are excluded and elute first.
The reason why larger molecules elute first is because they are too large to enter the pores of the resin. As a result, they take a longer path through the column, traveling arund the beads rather than through them. This results in a longer retention time for larger molecules, causing them to elute later than smaller molecules.
Another factor that affects the elution order of molecules is their shape. Molecules that are more compact and spherical in shape can penetrate pores more easily than elongated or irregularly shaped molecules. Therefore, molecules with a more compact shape will elute later than molecules with a more extended shape, even if they have a similar molecular weight.
The elution order of molecules in size exclusion chromatography is determined by their ability to penetrate the pores of the stationary phase. Larger molecules are excluded and take a longer path through the column, resulting in a longer retention time and earlier elution of smaller molecules. Shape also plays a role in determining elution order, with more compact molecules eluting later than more elongated or irregularly shaped molecules.
The Impact of Molecular Weight on Elution Time
In the process of gel filtration chromatography, larger molecules are excluded from the pores of the beads, while smaller molecules can penetrate them and travel thrugh the beads. As a result, larger molecules have a shorter path to travel and are eluted first, while smaller molecules have a longer path to travel and are eluted later. This is due to the fact that larger molecules get stuck less often in the maze of pores running from bead to bead, whereas smaller molecules get stuck more often. Therefore, heavier molecules elute first, and lighter molecules elute later. This phenomenon is a result of the size exclusion effect in gel filtration chromatography.
Does Boiling Point Affect Elution Order?
Higher boiling points do not elute first. In fact, the opposite is true. Compounds with lower boiling points or higher volatility elute first during a chromatography separation. This is because chromatography separates compounds based on teir affinity to the stationary and mobile phases. Compounds with higher volatility have a weaker affinity to the stationary phase, meaning they spend less time interacting with it and elute more quickly. On the other hand, compounds with lower volatility have a stronger affinity to the stationary phase, meaning they spend more time interacting with it and elute more slowly. Therefore, it is important to consider the volatility of the compound when designing a chromatography separation method.
The Impact of Temperature on Compound Elution
Compounds elute faster at higher temperatures due to the impact of temperature on the vapor pressure of the liquid. Vapor pressure refers to the pressure exerted by the vapor molecules over the liquid surface. At higher temperatures, the vapor pressure of the liquid increases because the kinetic energy of molecules in the liquid increases, and more molecules can escape the liquid’s surface.
In the case of chromatography, elution is the process of separating the components of a mixture based on teir affinity for the stationary phase. The elution process involves the transfer of the components from the stationary phase to the mobile phase. The rate of elution is affected by various factors, including temperature.
When the temperature is increased, the vapor pressure of the liquid increases, leading to an increase in the concentration of the component in the vapor phase. This, in turn, leads to a decrease in the concentration of the component in the stationary phase and an increase in the rate of elution. Additionally, at higher temperatures, the viscosity of the liquid decreases, allowing for faster diffusion of the component through the stationary phase.
It is important to note that the effect of temperature on elution rate depends on the nature of the compound and the stationary phase. For example, in gas chromatography, increasing the temperature can cause the column to become too hot, leading to the decomposition of the compound and the loss of resolution. However, in liquid chromatography, increasing the temperature can improve the separation efficiency, especially for nonpolar compounds.
Compounds elute faster at higher temperatures due to the increase in vapor pressure and the decrease in viscosity of the liquid, leading to faster diffusion of the component through the stationary phase.
Comparison of Polar and Nonpolar Elution Rates
When it comes to chromatography, the polarity of the solvent plays a critical role in determining the elution time of compounds. In general, polar solvents tend to elute compounds faster than nonpolar solvents, regardless of the polarity of the compounds themselves.
This is because polar solvents have a higher affinity for polar compounds, which allows them to interact more strongly with the stationary phase of the chromatography column. As a result, the polar compounds spend less time interacting with the stationary phase and more time in the mobile phase, leading to faster elution times.
Nonpolar solvents, on the other hand, have a higher affinity for nonpolar compounds, which can case them to interact more strongly with the stationary phase. This leads to slower elution times for nonpolar compounds, even if they have a lower polarity than the polar compounds in the mixture.
It’s worth noting that the exact elution times for different compounds will depend on a variety of factors beyond just the polarity of the solvent, including the size and shape of the molecules, the type of stationary phase used, and the temperature and pressure conditions during the experiment.
While the polarity of the compounds being separated is important in chromatography, the polarity of the solvent can have an even greater impact on elution times. In general, polar solvents will elute compounds faster than nonpolar solvents, regardless of the polarity of the individual compounds.
Effect of Polar and Nonpolar Molecules on Elution Speed
Thin Layer Chromatography (TLC) is a widely used technique in chemistry to separate and identify different components of a mixture. In TLC, a small amount of the mixture is spotted onto a stationary phase, usually a thin layer of silica gel or alumina on a plate. Then, the plate is placed in a container with a mobile phase, which is a solvent that moves up the plate by capillary action, carrying the mixture components with it.
One of the main factors that affect the separation of the mixture components in TLC is the polarity of the molecules. Polar molecules have a net dipole moment due to the unequal distribution of electrons within the molecule, while nonpolar molecules have a symmetrical electron distribution and no net dipole moment.
In TLC, nonpolar molecules tend to move up the plate more rapidly than polar molecules. This is because the mobile phase used in TLC is usually a nonpolar or weakly polar solvent, such as hexane or ethyl acetate. Nonpolar molecules are more soluble in nonpolar solvents, so they are carried more easily and quickly up the plate by the mobile phase. As a result, nonpolar molecules have a higher Rf (retention factor) value, which is the ratio of the distance traveled by the molecule to the distance traveled by the mobile phase.
On the othr hand, polar molecules are less soluble in nonpolar solvents, so they tend to stick more strongly to the stationary phase, and thus move up the plate more slowly or not at all. Polar molecules have a lower Rf value, indicating that they are less mobile in the mobile phase.
To summarize, nonpolar molecules elute faster than polar molecules in TLC, as they are more soluble in nonpolar solvents and less likely to stick to the stationary phase. Polar molecules, on the other hand, have a lower mobility in the mobile phase and tend to stick to the stationary phase, resulting in a slower elution and a lower Rf value.

Order of Elution in GC Analysis
Gas chromatography is a commonly used analytical technique in chemistry for separating and analyzing mixtures of volatile compounds. In gas chromatography, the components of the mixture are separated based on their boiling points and their affinity to the stationary phase.
To answer the question, “Which of the following will be eluted first from GC analysis?”, it is important to understand that the elution order is based on the boiling points of the components in the mixture. The component with the lowest boiling point will elute first, followed by the component with the next lowest boiling point, and so on.
Out of the options given, methanol has the lowest boiling point of -64.7°C. Therefore, it will be eluted first in the gas chromatography analysis.
It is important to note that the elution order can also be influenced by other factors such as the type of stationary phase used and the flow rate of the carrier gas. However, in general, the boiling point is the primary factor that determines the elution order in gas chromatography.
In gas chromatography, the component with the lowest boiling point will be eluted first. In the givn options, methanol has the lowest boiling point and will therefore elute first in the analysis.
Conclusion
Liquid chromatography is a powerful analytical technique used in the separation and purification of complex mixtures. The stationary phase and mobile phase used in liquid chromatography play a critical role in determining the elution order of solutes. Polydimethyl siloxane is a commonly used stationary phase in gas chromatography, and its replacement with other substituents can increase the stationary phase’s polarity and selectivity.
In normal-phase liquid chromatography, the elution order of solutes is governed by polarity. The least polar analytes elute first, while more polar analytes are retained longer. The silica stationary phase interacts with more polar molecules, while the hexane mobile phase carries nonpolar molecules. The elution order of solutes can be reversed by using a reverse-phase stationary phase, where the more polar analytes will elute first.
Liquid chromatography has several advantages over other chromatographic techniques, including high resolution, sensitivity, and selectivity. It is widely used in various fields, including pharmaceuticals, biotechnology, environmental science, and food science.
Liquid chromatography is a powerful analytical technique that plays a critical role in the separation and purification of complex mixtures. Understanding the principles of liquid chromatography and the interactions beteen the stationary and mobile phases is essential for successful chromatographic separations. The versatility and wide range of applications make liquid chromatography a valuable tool in various fields of science and industry.
