Chlorine is a chemical element with the symbol Cl and atomic number 17. It is a highly reactive and corrosive gas that belongs to the halogen group on the periodic table. Chlorine is widely used in a variety of industrial processes and is an important ingredient in the production of bleach, plastics, and many other products.
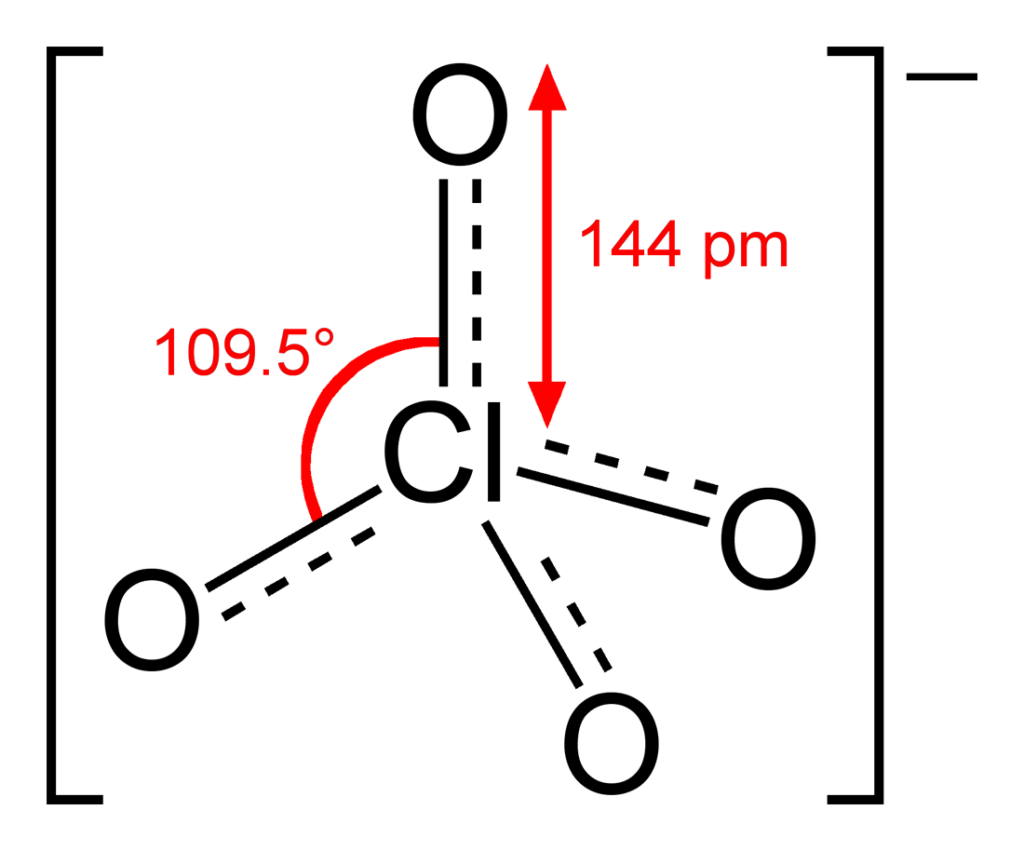
One of the most common forms of chlorine is perchlorate, which is a compound made up of one chlorine atom and four oxygen atoms. Perchlorate is used in a variety of applications, including rocket fuel, fireworks, and as a component in some types of batteries.
When we talk about the formal charge of an atom, we are referring to the difference between the number of valence electrons an atom has and the number of electrons it actually has in a molecule. The formal charge helps us understand the distribution of electrons in a molecule and is an important concept in chemistry.
In the case of perchlorate, the formal charge on the central chlorine atom is +3, while each of the four oxygen atoms has a formal charge of -1. This means that the chlorine atom is carrying a positive charge, while the oxygen atoms are carrying negative charges.
There are a few different ways to calculate the formal charge of an atom, but one common method is to use the follwing formula:
Formal charge = valence electrons – (lone pair electrons + 1/2 bonding electrons)
Using this formula, we can calculate the formal charge on the chlorine atom in perchlorate as follows:
Valence electrons for chlorine = 7
Lone pair electrons on chlorine = 0
Bonding electrons with oxygen atoms = 8 (4 oxygen atoms with 2 bonding electrons each)
Formal charge = 7 – (0 + 8/2) = +3
It’s important to note that the formal charge on an atom does not necessarily reflect its actual charge or its behavior in a chemical reaction. The formal charge is simply a tool we use to understand the distribution of electrons in a molecule.
The formal charge of chlorine in perchlorate is +3, while each of the oxygen atoms has a formal charge of -1. This helps us understand the distribution of electrons in the molecule and is an important concept in chemistry.
Formal Charge of Chlorine
The formal charge on an atom is a way to determine its electron distribution in a molecule. To calculate the formal charge of an atom, we need to subtract the number of non-bonded electrons and half of the bonded electrons from the total number of valence electrons.
In the case of chlorine, which is a halogen, it has seven valence electrons. In the molecule where chlorine is the central atom and surrounded by four oxygen atoms, it forms four single bonds with oxygen atoms, and each oxygen atom also has two lone pairs of electrons.
To calculate the formal charge of chlorine, we need to follow the formula:
Formal charge = valence electrons – non-bonded electrons – half of bonded electrons
Using this formula, we get:
Formal charge on chlorine = 7 – 0 – (4 x 2)/4 = +3
Therefore, the formal charge on the central chlorine atom in the gven molecule is +3.
It is worth noting that the formal charge does not necessarily reflect the actual charge on the atom. It is merely a way to determine electron distribution in a molecule. The actual charge on an atom is affected by various factors, such as electronegativity, bond polarity, and resonance.
Source: youtube.com
Formal Charge of Oxygen Atom in Clo4
Chlorate ion (ClO4-) is a polyatomic ion that consists of one central chlorine atom surrounded by four oxygen atoms. To determine the formal charge on the oxygen atom in ClO4-, we frst need to understand what formal charge is.
Formal charge is a measure of the distribution of electrons in a molecule or ion. It is calculated by subtracting the number of valence electrons on an atom in its neutral state from the number of valence electrons it has in the molecule or ion, taking into account the number of bonds and lone pairs of electrons that atom has.
The formula for calculating the formal charge on an atom can be expressed as:
Formal charge = (number of valence electrons on the neutral atom) – (number of lone pair electrons) – (1/2 x number of bonding electrons)
Applying this formula to the oxygen atom in ClO4-, we can see that each oxygen atom is bonded to the central chlorine atom via a single bond and has three lone pairs of electrons.
The number of valence electrons on an oxygen atom is 6, so:
Formal charge = 6 – 3 – (1/2 x 6) = -1
Therefore, each oxygen atom in ClO4- has a formal charge of -1. It is important to note that the sum of the formal charges on all atoms in a molecule or ion should add up to the overall charge of the molecule or ion. In this case, there are four oxygen atoms with a formal charge of -1 each, and one chlorine atom with a formal charge of +1, making the overall charge of ClO4- equal to -1.
Calculating the Formal Charge
In chemistry, formal charge is a way to determine the charge of an atom in a molecule. It is a useful tool for predicting the reactivity and behavior of molecules. To calculate the formal charge of an atom, you need to follow a specific formula.
The formula to calculate formal charge is:
FC = VE – [LPE – ½(BE)]
Where FC is the formal charge, VE is the number of valence electrons on the free atom, LPE is the number of lone pair electrons on the atom in the molecule, and BE is the number of bonding (shared) electrons around the atom in the molecule.
To find the formal charge of an atom, follow thee steps:
1. Determine the number of valence electrons on the free atom. This is the number of electrons in the outermost shell of the atom.
2. Count the number of lone pair electrons on the atom in the molecule. Lone pair electrons are electrons that are not involved in bonding and are located on the atom itself.
3. Count the number of bonding electrons around the atom in the molecule. Bonding electrons are electrons that are shared between two atoms.
4. Subtract the sum of the number of lone pair electrons and half the number of bonding electrons from the number of valence electrons. This will give you the formal charge of the atom.
It is important to note that the sum of the formal charges of all atoms in a molecule should add up to the total charge of the molecule. If the total charge of the molecule is zero, then the sum of the formal charges of all atoms in the molecule should also be zero.
The formal charge of an atom can be calculated using the formula FC = VE – [LPE – ½(BE)]. By following the steps outlined above, you can determine the formal charge of any atom in a molecule.
Number of Electrons in the Lewis Structure of Perchlorate Ion ClO4-
The Lewis structure of the perchlorate ion (ClO4 -) displays a total of 32 electrons. This can be determined by adding up the valence electrons of each atom in the molecule. Chlorine has 7 valence electrons, oxygen has 6 valence electrons, and there are four oxygen atoms in the perchlorate ion, giving a total of 7 + 4(6) = 31 valence electrons. The negative charge on the ion signifies the addition of one extra electron, resulting in a total of 32 electrons in the Lewis structure. It is essential to consider the electron count as it helps to understand the bonding and geometry of the molecule.
Bond Order of ClO4 Negative
The bond order of ClO4 negative can be determined trough the calculation of the average number of bonds between the atoms in the molecule. In the case of ClO4 negative, which is also known as the perchlorate ion, we can count the number of resonance structures it can make to determine its bond order.
Perchlorate ion has four oxygen atoms bonded to a central chlorine atom, and each oxygen atom has a single negative charge. The Lewis structure of the molecule shows that each oxygen atom is bonded to the chlorine atom through a single bond, and two of the oxygen atoms are also bonded to each other through a double bond.
By counting the number of resonance structures that the perchlorate ion can make, we can determine that the bond order of the molecule is 1.75. This indicates that the bonds between the atoms in the molecule are not perfectly single or double bonds but have a partial double bond character.
Understanding the bond order of ClO4 negative is important for predicting its chemical behavior and reactivity in various chemical reactions.
Conclusion
Chlorine is a highly reactive chemical element that is commonly used in a variety of industrial applications. It is a member of the halogen family and is located in group 17 of the periodic table. Chlorine is a greenish-yellow gas with a pungent odor and is highly toxic in its pure form. However, it is also an essential element for life and is used in the production of a wide range of products, including plastics, solvents, and cleaning agents.
Chlorine is a fascinating and versatile element that has a wide range of applications in industry and daily life. While it can be dangerous in its pure form, it is also an essential component of many usful products and processes. Understanding the properties and behavior of chlorine is important for anyone working in chemistry, materials science, or related fields. By continuing to explore the properties and potential applications of chlorine, we can continue to improve our understanding of this important element and its role in our world.
