Fluorine, a highly reactive and corrosive element, exists as a diatomic molecule (F2) in its natural state. Despite being a non-polar molecule, F2 exhibits some unique intermolecular forces that make it an interesting subject of study.
The intermolecular forces present in F2 are known as van der Waals forces. These forces arise due to temporary fluctuations in the electron density of the molecule, causing the instantaneous formation of temporary dipoles. These temporary dipoles induce similar dipoles in neighboring molecules, leading to a weak attractive force between them.
Van der Waals forces are the weakest intermolecular forces and are present in all molecules, but for F2, they are the only intermolecular force present. This is because F2 is a non-polar molecule, meaning it has no permanent dipole moment due to the equal sharing of electrons between the two fluorine atoms.
Despite being weak, van der Waals forces play a crucial role in determining the physical properties of F2. For instance, these forces are responsible for the low boiling point of F2 (-188°C) and its low melting point (-219°C). The weak intermolecular forces make it easy to overcome the attractive forces between molecules, leading to a low energy requirement for phase changes.
It is interesting to note that the absence of strong intermolecular forces in F2 makes it a highly reactive molecule. The weak van der Waals forces make it easy for other molecules to approach F2 and participate in chemical reactions. This reactivity is why F2 is a powerful oxidizing agent and is widely used in the chemical industry.
The intermolecular forces present in F2 are purely van der Waals forces, which arise due to temporary fluctuations in the electron density of the molecule. Despite being weak, these forces play a crucial role in determining the physical properties of F2 and make it a highly reactive molecule. Understanding these intermolecular forces is crucial for studying the properties and reactivity of F2.
Intermolecular Forces Present in Fluorine Gas
Fluorine gas is composed of diatomic molecules (F2) that are non-polar, meaning they have an equal distribution of electrons and no net dipole moment. As a result, the intermolecular forces between fluorine molecules are very weak and are primarily due to van der Waals forces. These forces arise from temporary dipoles that occur when the electron distribution in one molecule is slightly distorted by the presence of another molecule. While van der Waals forces are relatively weak, they are still strong enough to keep the fluorine molecules together in a gas phase at low temperatures and high pressures.
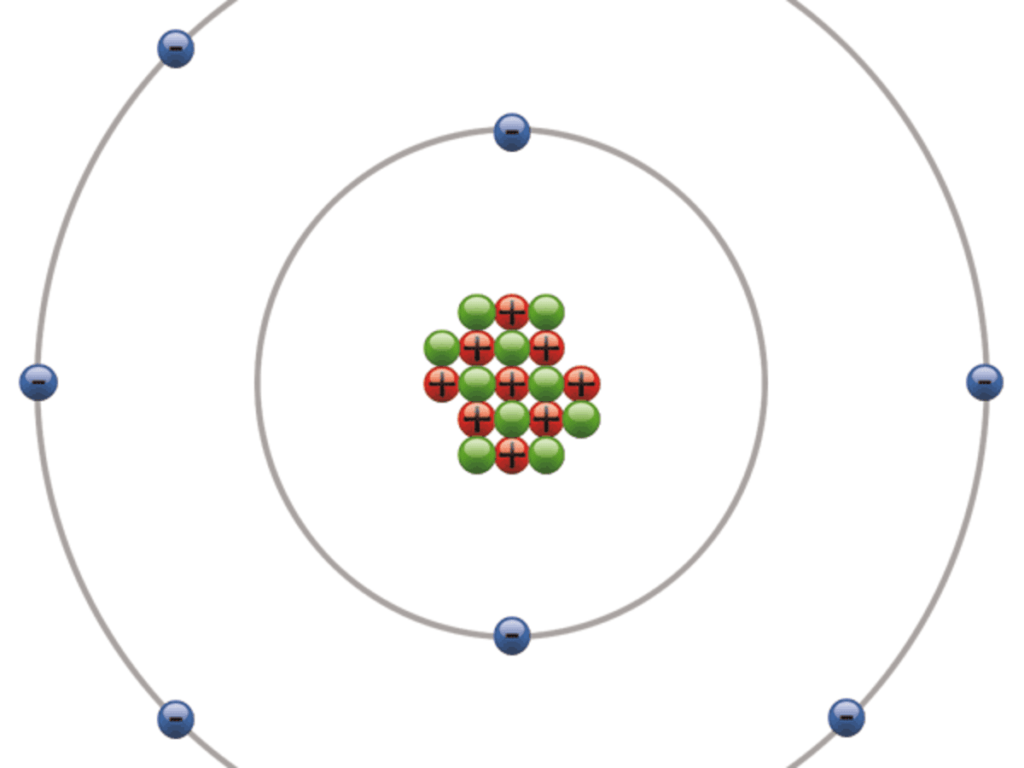
Intermolecular Force of F2
The type of intermolecular force that is greatest for F2 is known as London Dispersion Forces (LDFs). These forces arise from temporary dipoles that occur in molecules due to the random movements of electrons. In F2, the electrons are distributed evenly and symmetrically around the molecule, which results in a non-polar molecule, and hence, weak intermolecular forces. However, despite this, F2 is sill capable of experiencing LDFs, which are the weakest of all intermolecular forces. They increase with the size of the molecule, which means that the larger the molecule, the greater the LDFs. Hence, while F2 has weak intermolecular forces, they are still present and contribute to the overall behavior of the substance.
Presence of Hydrogen Bonding in F2
Hydrogen bonding can be present in F2 molecules. However, the strength of hydrogen bonding in F2 is weaker than that of molecules containing oxygen, nitrogen, or fluorine atoms. This is because fluorine is more electronegative than other halogens, which makes the bond between hydrogen and fluorine more polarized, allowing for some hydrogen bonding to occur. However, the strength of hydrogen bonding in F2 is still considerably weaker than in molecules containing oxygen or nitrogen atoms.
Does F2 Exhibit Dipole-Dipole Attractions?
F2 does not have dipole-dipole attractions. This is becuse the two fluorine atoms in F2 molecule are identical and have the same electronegativity. As a result, the electrons are shared equally between the two atoms, leading to a nonpolar covalent bond with no separation of charges. Dipole-dipole attractions arise due to the partial charges of polar molecules, which have an uneven distribution of electrons. Since F2 is a nonpolar molecule, it does not have any permanent dipole moment, and hence, no dipole-dipole attractions. However, F2 can exhibit London dispersion forces, which are weak intermolecular forces that arise due to the temporary fluctuations in electron density within the molecule.
Does Fluorine Exhibit Dipole-Dipole Interactions?
Fluorine has dipole-dipole interactions due to its highly electronegative nature. Fluorine atom attracts electrons towards itself in a covalent bond, creating a partial negative charge on the fluorine and a partial positive charge on the other atom. When two fluorine atoms are bonded together, they exhibit a significant dipole moment due to the large electronegativity difference between the two atoms. This results in a dipole-dipole interaction between the two fluorine atoms, which can affect the physical and chemical properties of the molecule.

Source: youtube.com
Bonding Type of Fluorine Gas
Fluorine gas forms a covalent bond. A covalent bond is a type of chemical bond in which two atoms share electrons to form a stable molecule. In the case of fluorine gas, the two fluorine atoms share two electrons to form a stable F2 molecule. This covalent bond is very strong, which is why fluorine gas is highly reactive and capable of forming bonds with many other elements.
Bonding in F2
Fluorine gas (F2) is a diatomic molecule, meaning it consists of two atoms of fluorine that are chemically bonded together. The bond that holds the two atoms of fluorine together is a covalent bond. In covalent bonding, atoms share electrons in order to achieve a more stable electron configuration. In the case of F2, each fluorine atom has seven valence electrons, and they share a pair of electrons to form a single covalent bond between them. The shared pair of electrons is attracted to both nuclei, creating a stable molecule. F2 is a great exmple of a covalently bonded molecule.
Polarity of F2 Bond
The bond btween the two F atoms in the fluorine molecule, F2, is non-polar covalent. This means that the two atoms share the electrons equally, resulting in a balanced distribution of charges and no partial charges on the atoms. Non-polar covalent bonds occur when two atoms with similar electronegativities share electrons to form a stable molecule. In the case of F2, both fluorine atoms have the same electronegativity, resulting in a non-polar covalent bond. It’s important to note that the polarity of a bond depends on the difference in electronegativity between the atoms involved, and in the case of F2, the electronegativity difference is negligible.
The Role of London Dispersion Forces in F2 Molecules
F2 has only London dispersion forces. This is because F2 is a non-polar molecule, which means that it has no permanent dipole moment. London dispersion forces, also known as induced dipole-induced dipole forces, are the weakest intermolecular forces that exist between non-polar molecules. They arise due to temporary fluctuations in the electron distribution of the molecule, which create a temporary dipole moment. These temporary dipoles can induce similar dipoles in neighboring molecules, leading to attractive forces between them. Since F2 is a non-polar molecule, it has no other intermolecular forces such as dipole-dipole or hydrogen bonding, and therefore, only London dispersion forces exist between its molecules.
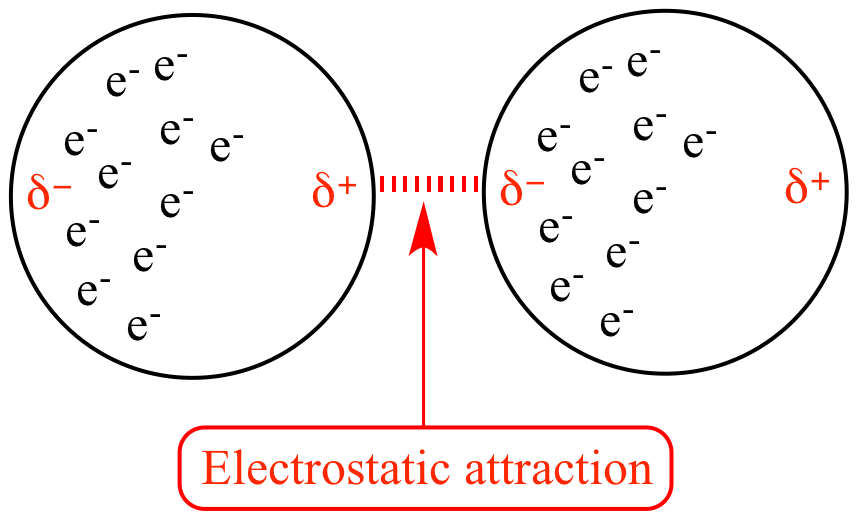
The Intermolecular Forces of F2
F2 has weak intermolecular forces. This is due to several factors, including the small size of the fluorine atoms and the low number of electrons they have. The small size of the atoms means that the London dispersion forces (LDF), whch are the intermolecular forces that arise from temporary dipoles induced by the motion of electrons, are weaker compared to larger atoms. Additionally, the low number of electrons means that there is less opportunity for other types of intermolecular forces, such as dipole-dipole interactions or hydrogen bonding, to occur. Therefore, the overall intermolecular forces between F2 molecules are relatively weak, which results in low boiling and melting points for the compound.
Conclusion
The intermolecular forces present in fluorine (F2) are weak van der Waals forces due to the non-polar nature of the molecule. However, F2 is capable of forming hydrogen bonds with strongly electronegative elements such as oxygen, fluorine, and nitrogen. These hydrogen bonds are the strongest intermolecular force and require a significant amount of energy to break. When compared to other molecules such as HCl, which has permanent dipole-dipole attractions, F2 has weaker intermolecular forces. Therefore, F2 has a lower boiling point compared to HCl. understanding the intermolecular forces present in F2 is crucial in predicting its physical and chemical properties.
