Osmosis is the process by which water moves across a semi-permeable membrane from an area of high water concentration to an area of low water concentration. This movement of water is caused by the difference in solute concentration on either side of the membrane. But the question is, does osmosis require energy?
The answer is no, osmosis does not require energy. ATP, the molecule that provides energy for cellular processes, is not required for osmosis to occur. Instead, osmosis is a passive process that occurs naturally due to the random movements of water molecules.
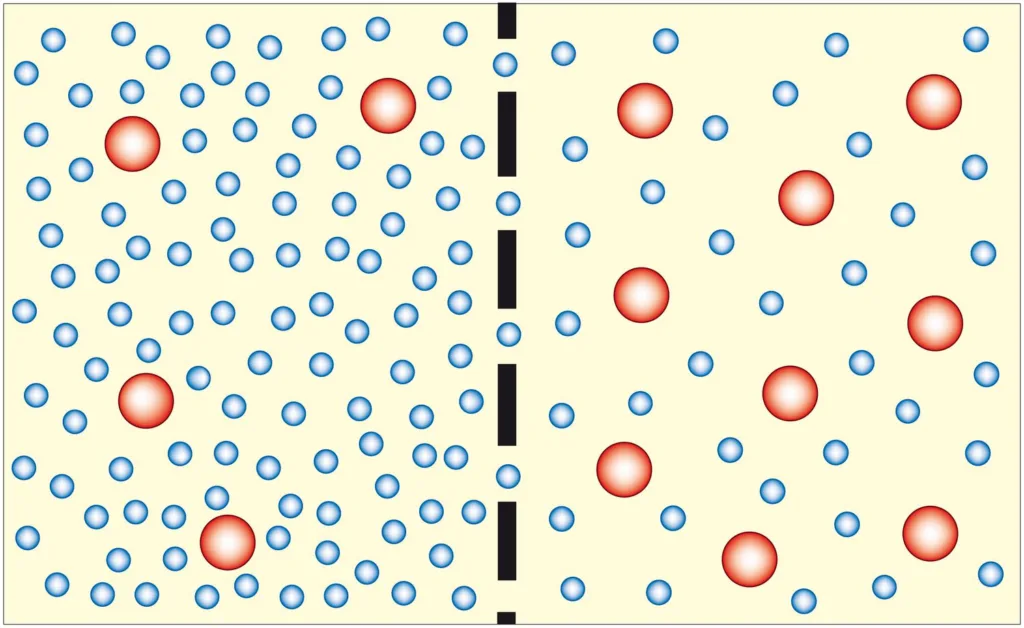
In order for osmosis to occur, the membrane must be permeable to water, but impermeable to the solute. This means that the solute cannot pass through the membrane, while the water molecules can. Additionally, there must be a difference in solute concentration on either side of the membrane, creating a concentration gradient that drives the movement of water.
Once the concentration gradient is established, water molecules will diffuse across the membrane from the side with high water concentration to the side with low water concentration. This diffusion is driven by the natural movement of water molecules, which are constantly in motion due to their kinetic energy.
While osmosis itself does not require energy, there are factors that can affect the rate at which it occurs. For example, the presence of channel proteins in the membrane can increase the rate of osmosis by providing a larger pathway for water molecules to pass through. Similarly, if the solute concentration gradient is very steep, osmosis may occur more quickly than if the gradient is more gradual.
Osmosis is a passive process that occurs naturally due to the movement of water molecules. It does not require energy, but is instead driven by the concentration gradient of solutes on either side of the membrane. By understanding the principles behind osmosis, we can better understand how water and oter substances move in and out of cells, and how this process can be manipulated for various applications, such as water filtration or drug delivery.
Does Osmosis Require ATP for Energy?
Osmosis is the process by which water molecules move across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. This movement of water occurs to balance the concentration of solutes on either side of the membrane. Unlike active transport, which requires the use of energy in the form of ATP, osmosis is a passive process that does not require any additional energy beyond the inherent kinetic energy of the water molecules themselves. Thus, it can be concluded that osmosis does not require the use of energy ATP.

Source: setapht.com
What Is Required By Osmosis?
Osmosis is a process by which water molecules move from an area of high water concentration to an area of low water concentration through a selectively permeable membrane. For osmosis to occur, the membrane must be permeable to water, allowing it to pass through easily, but impermeable to the solute, which cnnot cross the membrane. Furthermore, there must be a concentration gradient of the solute, meaning that the solute concentration must be different on the two sides of the membrane. The movement of water molecules continues until the concentration of water is equal on both sides of the membrane, creating equilibrium. Therefore, the key factors required for osmosis are a selectively permeable membrane, a solute, a concentration gradient, and water molecules.
The Reason Why Osmosis and Diffusion Do Not Require Energy
Osmosis and diffusion are two fundamental processes that occur in cells and tissues of living organisms. Both of these processes do not require any energy to take place. The reason behind this is that the substances move down the concentration gradient, from high to low concentration. In other words, the net flow of the substances is caused by the random movement of the substances owing to the energy level of the substances.
Diffusion is the process by which molecules move from an area of high concentration to an area of low concentration. This process occurs spontaneously and is driven by the natural tendency of molecules to move from areas of high concentration to areas of low concentration. Diffusion is responsible for the movement of oxygen and carbon dioxide in and out of cells, the movement of nutrients and waste products across cell membranes, and the distribution of substances within tissues and organs.
Osmosis, on the other hand, is the diffusion of water molecules across a selectively permeable membrane. This process occurs when thee is a difference in the concentration of solutes on either side of the membrane. Water molecules move from an area of high water concentration (low solute concentration) to an area of low water concentration (high solute concentration) until the concentration of solutes is equal on both sides of the membrane. This process is also driven by the natural tendency of molecules to move from areas of high concentration to areas of low concentration.
Both diffusion and osmosis do not require energy because they are driven by the natural tendency of molecules to move from areas of high concentration to areas of low concentration. This movement is caused by the random motion of the molecules and the energy level of the substances.
The Energy and Protein Requirements of Osmosis
Osmosis is a passive process that does not require energy or protein to occur. It is the movement of water molecules across a selectively permeable membrane from an area of low solute concentration to an area of high solute concentration. Since water molecules are small enugh to pass through the membrane, osmosis can happen directly through the membrane without the need for a transport protein. However, channel proteins can be used to increase the rate at which osmosis occurs. These proteins create a water-filled channel through the membrane, providing a pathway for water molecules to move more quickly. osmosis is a passive process that does not require energy or protein, but channel proteins can be used to enhance its rate.
Conclusion
Osmosis does not require energy in the form of ATP from the cell. Instead, it is driven by the concentration gradient of the solute on either side of the membrane. Osmosis occurs when the membrane is selectively permeable to water but impermeable to the solute, and the concentration of the solute is different on the two sides of the membrane. Although no transport proteins are required for osmosis to occur, channel proteins can increase the rate at which it happens. Therefore, osmosis is a passive process, and the movement of water across the membrane occurs spontaneously without the need for any expenditure of energy.
