Ozone, with its chemical formula O3, is a molecule that has been studied extensively due to its role in the Earth’s atmosphere. One of the most interesting features of this molecule is its ability to exhibit resonance, which is a phenomenon that occurs when the electrons in a molecule are delocalized and can move freely between certain atoms.
Resonance occurs in O3 due to the presence of a double bond and a single bond between the three oxygen atoms. The double bond is formed between two of the oxygen atoms, whle the third oxygen is bonded to one of the other oxygen atoms through a single bond. This arrangement of bonds creates a situation where the electrons in the molecule can move freely between the different atoms, leading to resonance.
The delocalization of the pi electrons in O3 causes resonance, and as a result, two resonance structures are drawn for this molecule. These structures are crucial in understanding the behavior of ozone and its reactivity with other molecules. In the first resonance structure, one of the oxygen atoms has a double bond with the central oxygen atom, while the other oxygen atom has a single bond with the central oxygen. In the second resonance structure, the double bond moves to the other side of the molecule, creating a double bond between two different oxygen atoms.
It is important to note that the net charge of resonance structures will be the same, as the total number of electrons in the molecule remains constant. However, the distribution of electrons is different in each resonance structure, which can have important implications for the reactivity of the molecule.
Ozone is a molecule that exhibits resonance due to the delocalization of its pi electrons. This leads to the formation of two resonance structures that are important for understanding the behavior of the molecule. The study of ozone and its resonance structures is crucial for understanding its role in the Earth’s atmosphere and its impact on climate change.
Number of Resonance Structures of O3
Ozone (O3) is a molecule that exhibits resonance, which means that its electrons can be delocalized over multiple atoms. As a result, O3 can have more than one valid Lewis structure, and we refer to these structures as resonance structures.
When we draw the Lewis structure of O3, we find that it has a double bond between one oxygen atom and the central oxygen atom, while the other oxygen atom has a single bond. However, we can also draw a resonance structure where the double bond is between the other oxygen atom and the central oxygen atom, and the single bond is on the opposite side.
Therefore, O3 can make two resonance structures, which means that the electrons in the molecule are not localized to just one configuration but rather are distributed aross two equally valid structures. This concept of resonance is important in understanding the properties of molecules and their reactivity.
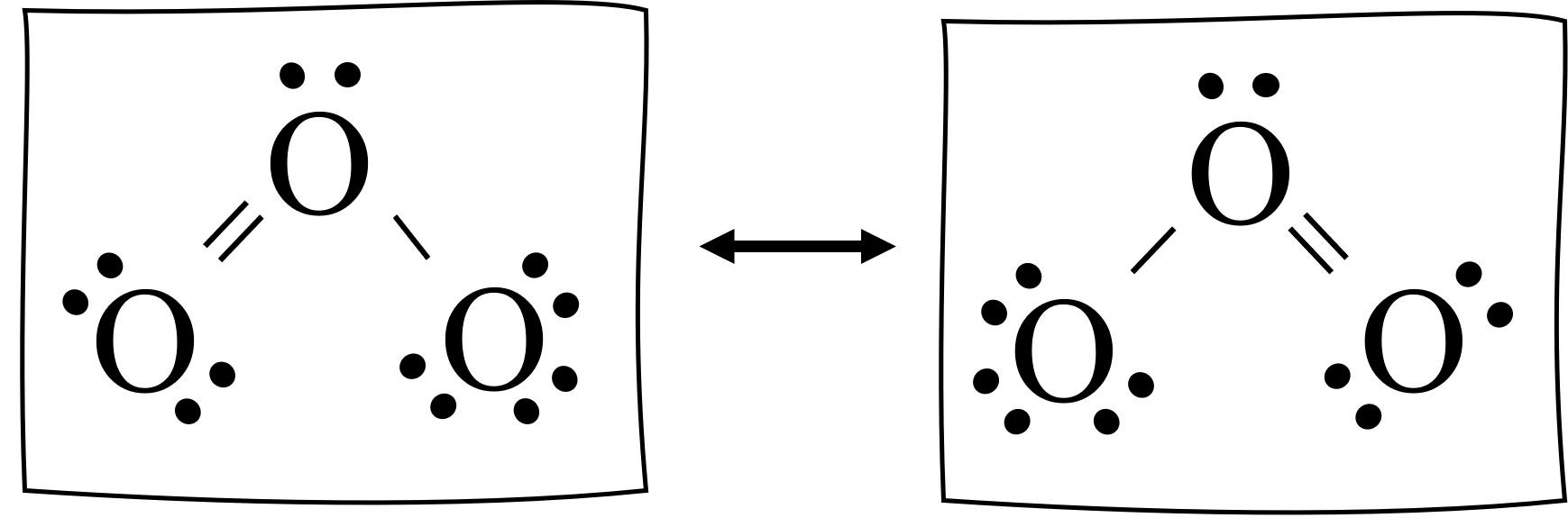
Source: learnwithdrscott.com
The Resonance of O3
Ozone (O3) has resonance because its actual electronic structure is a hybrid of several resonance structures. The resonance structures arise from the delocalization of electrons in the molecule. The oxygen atoms in ozone are sp2 hybridized, with one lone pair of electrons in a p orbital. The two oxygen atoms on eiter side of the central oxygen atom are double-bonded to it, with one of the pi bond electrons being delocalized over all three atoms. This delocalization creates a resonance structure in which the double bond is shifted to the other side of the central oxygen atom, resulting in a molecule with a single bond on one side and a double bond on the other.
The resonance stabilization energy of ozone is the energy released when the actual electronic structure of the molecule is formed from its resonance structures. This energy is due to the delocalization of electrons over all three oxygen atoms, leading to a more stable electronic configuration. The resonance structures of ozone involve a molecular orbital extending over all three oxygen atoms, which is formed from three atom-centered p orbitals. This molecular orbital is responsible for the delocalization of electrons and the resonance stabilization energy of ozone.
Ozone has resonance because the delocalization of electrons in the molecule creates several resonance structures, leading to a more stable electronic configuration. The molecular orbital extending over all three oxygen atoms is responsible for the delocalization of electrons and the resonance stabilization energy of ozone.
Number of Resonances of Ozone
Ozone, which is a triatomic molecule composed of three oxygen atoms, has two resonating structures. This means that the electrons in the molecule can be delocalized between two different arrangements of the atoms, giving rise to two possible resonance structures. The two resonance structures of ozone are equivalent and contribute equally to the overall electronic structure of the molecule. It is worth noting that the net charge of resonance structures will always be the same, and the concept of resonance is a crucial tool in understanding the behavior and properties of many molecules in chemistry.
Exploring Resonance in Carbon Dioxide
There is resonance in CO2. Resonance is a phenomenon that occurs when two or more Lewis structures can be drawn for a molecule that differ only in the arrangement of electrons. In CO2, there are three resonance structures that can be drawn by shifting the double bonds between the carbon and oxygen atoms. These structures have equal importance in describing the actual electronic distribution in the molecule. However, the structure in whch both oxygen atoms have a double bond with carbon is the major contributor to the resonance hybrid, as it satisfies the octet rule for all atoms and has the lowest energy.
Why O3 Does Not Form a Ring
Ozone (O3) does not form a ring because of a phenomenon known as electronic repulsion. Ozone is composed of three oxygen atoms, each with six valence electrons. In order to form a ring, these electrons would have to bend at 60 degrees, which is not a favorable arrangement due to the repulsion between the negatively charged electrons. This repulsion makes the molecule very unstable and unlikely to form a ring structure. Instead, the oxygen atoms in ozone form a bent or V-shaped molecule, with the two oxygen-oxygen bond angles measuring approximately 117 degrees. This configuration alows for the valence electrons to be distributed more evenly and reduces the electronic repulsion between them, resulting in a more stable molecule. Therefore, the electronic repulsion is the main reason why ozone does not form a ring.
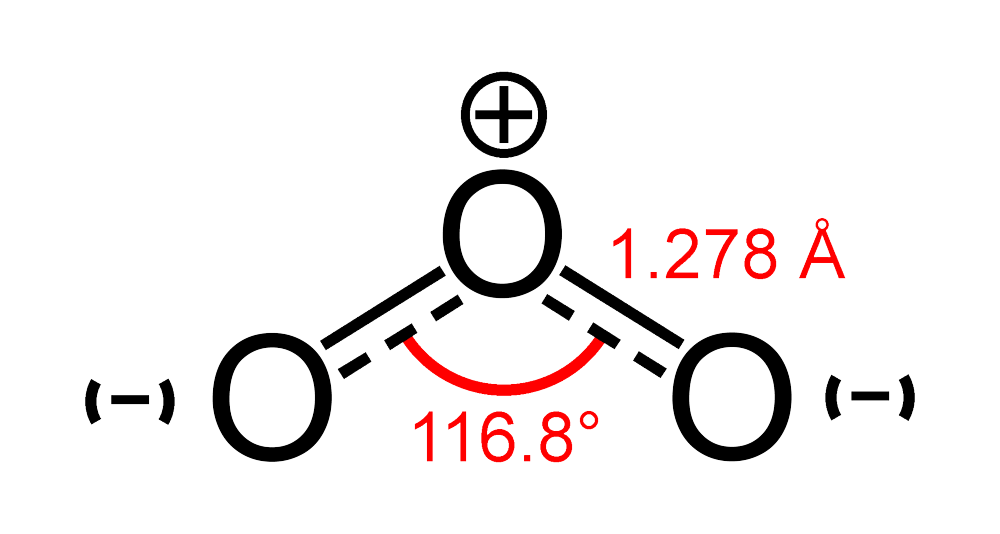
The Reactivity of O3
Ozone, or O3, is a highly reactive gas due to the unstable nature of its chemical bonds. The molecule is formed by three oxygen atoms, and the bonds holding them toether are relatively weak compared to those in other molecules, such as O2. This means that ozone is constantly breaking apart and reforming, creating a highly reactive environment. Additionally, ozone is an oxidant, meaning it readily accepts electrons from other molecules, which can cause chemical reactions to occur. Its reactivity makes it useful in a variety of applications, such as air and water purification, but it can also be harmful to living organisms if present in high concentrations. the unstable bonds and oxidizing properties of ozone contribute to its high reactivity.
Does O2 Have Resonance Structures?
O2 (molecular oxygen) does not exhibit resonance because it contains only two atoms, and there is no possibility for the double bond to be located in different positions. Resonance occurs when there are multiple equivalent ways to draw the Lewis structure of a molecule, but this is not the case with O2. The double bond in O2 is formed by the sharing of two electrons between the two atoms, and this bond is quite stable due to the high electronegativity of oxygen. Therefore, there is no need for resonance to occur in O2 to stabilize the molecule.
Does O3 Follow the Octet Rule?
Ozone (O3) obeys the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons in order to achieve a stable electron configuration with a full outer valence shell conaining eight electrons. In the case of ozone, each oxygen atom has six valence electrons, and by sharing electrons with each other, they are able to complete their octets and achieve a stable electron configuration. The resulting molecule has a total of 18 valence electrons, with each oxygen atom contributing six electrons and the lone pairs on the central oxygen atom contributing the remaining six. Although the two oxygen atoms in ozone have formal charges of +1 and -1, the overall charge of the molecule is zero, and all oxygen atoms follow the octet rule.
Does Water Have Resonance?
H2O does not have resonance. The concept of resonance occurs when a molecule has multiple equivalent structures that can be drawn by shifting electrons around. However, in the case of H2O, it contains two O-H bonds, which satisfy the valencies of both the oxygen and hydrogen atoms. Hence, there is no possibility of shifting electrons or any other equivalent structures. Therefore, H2O does not exhibit the phenomenon of resonance.
Does Hydrogen Sulfide Have Resonance?
H2S does not have resonance. Resonance occurs when a molecule can be represented by two or more Lewis structures that differ only in the placement of electrons. However, in the case of H2S, the molecule consists of a central sulfur atom bonded to two hydrogen atoms, and the lone pairs of electrons on the sulfur atom. There is no possibility for electron delocalization or movement between different positions, as there is no other possible arrangement of the atoms or electrons that would produce a lower energy state. Thus, H2S does not exhibit resonance.
Can NO2 Exhibit Resonance?
Resonance is possible in NO2. The NO2 molecule has a structure with a double bond betwen the nitrogen atom and one of the oxygen atoms, and a single bond between the nitrogen atom and the other oxygen atom. This arrangement creates two resonance structures for the molecule. In one structure, the nitrogen atom has a positive charge and the oxygen atoms have negative charges. In the other structure, the nitrogen atom has a lone pair of electrons and one of the oxygen atoms has a double bond with the nitrogen atom. The two resonance structures contribute to the overall stability of the molecule, making it more stable than if only one structure were present. Therefore, resonance is an important factor in the properties and behavior of NO2.
Does Ammonia (NH3) Exhibit Resonance?
NH3 (ammonia) does not have resonance. Resonance is a phenomenon that occurs when a molecule can have different possible structures, which are represented by resonance structures. These structures differ only in the placement of electrons, and they contribute to the overall stability of the molecule.
In the case of NH3, the nitrogen atom is sp3 hybridized, meaning that it has four electron pairs arond it, three of which are bonded to hydrogen atoms. The fourth pair of electrons is a lone pair, which is not involved in any bonding. This lone pair is localized on the nitrogen atom and cannot participate in resonance. Additionally, NH3 does not have any π bonds, which are required for resonance to occur. Therefore, NH3 is not capable of exhibiting resonance.
Conclusion
O3 or ozone exhibits resonance due to the delocalization of pi electrons. This phenomenon leads to the formation of two resonating structures, where the positions of electrons are distributed over the three oxygen atoms. The resonance structures of ozone involve molecular orbitals extending over all three oxygen atoms, formed from three atom-centered pz orbitals. The net charge of the resonance structures of ozone remains the same. Therefore, we can safely conclude that O3 does exhibit resonance, and this phenomenon is crucial in understanding the chemical behavior and properties of ozone.
