Helium is one of the most fascinating elements of the periodic table. It is a noble gas and belongs to Group 18. Helium has a unique electron configuration, which makes it stable and unlikely to gain or lose electrons.
The electron configuration of helium is 1s2, which means it has two electrons in the frst energy level. The first energy level can hold a maximum of two electrons, and since helium has two electrons, it has a complete shell. This makes it a very stable element that does not readily accept any extra electrons nor join with anything to make covalent compounds.
So, does helium gain or lose electrons? The answer is no. Helium is a non-metal and does not gain or lose electrons to form ions. In fact, it is one of the few elements that exist as a single atom rather than a molecule. Helium atoms are neutral, meaning they have an equal number of positively charged protons and negatively charged electrons.
However, it is possible to ionize helium by removing one or both of its electrons. When an electron is removed from a helium atom, it becomes a positive ion. This is because the number of positively charged protons now exceeds the number of negatively charged electrons. The resulting ion is called a helium ion or a alpha particle.
On the other hand, if an extra electron is added to a helium atom, it becomes a negative ion. This is because the number of negatively charged electrons now exceeds the number of positively charged protons. However, this is a highly unlikely scenario, as helium is a noble gas and does not readily accept extra electrons.
Helium does not gain or lose electrons in its natural state. It is a stable element that does not readily react with other elements or form compounds. Its unique electron configuration makes it a fascinating element that has many uses in various industries, including medical, scientific, and industrial applications.
Can Helium Lose Electrons?
Helium is a noble gas and belongs to the Group 18 elements in the periodic table. It has two electrons in its outermost shell, which makes it a very stable element. Due to its stability, helium is unlikely to lose or gain electrons, and it does not readily form chemical bonds with other elements. Therefore, it can be concluded that helium cannot lose electrons in a chemical reaction.
Does Helium Become an Ion by Losing or Gaining Electrons?
Helium can both lose or gain electrons to become an ion, but it is more common for helium to lose two electrons and form a positive ion. This is because helium has a stable electron configuration with two electrons in its outermost energy level, making it energetically favorable to lose tose two electrons and achieve a stable configuration with a full outer energy level. However, under certain conditions such as in the presence of strong electric fields or in collisions with other particles, helium can also gain electrons and form a negative ion. whether helium loses or gains electrons to become an ion depends on the specific environment and conditions it is exposed to.
Does Helium Gain or Lose Electrons?
Helium is a noble gas and has a completely filled outer shell of electrons with two electrons in it. As a result, it does not readily give away or accept any electrons from other atoms. This makes helium a chemically stable element, and it does not form ions or participate in any chemical reactions to form covalent compounds. Therefore, helium does not give or take electrons, as it has no tendency to form chemical bonds with other atoms.
Gaining and Losing Electrons
When two elements form an ionic compound, one element will typically gain electrons while the other element will lose electrons. This is because the goal of forming an ionic bond is for both elements to achieve a more stable electron configuration. The element with a higher electronegativity, which is the ability to attract electrons, will typically gain electrons. This is because it has a greater pull on the electrons and can hold them closer to its nucleus. The element with a lower electronegativity will typically lose electrons, as it has a weaker pull on the electrons and is more willing to give them up. However, it is important to note that tere are exceptions to this general rule and the exact behavior of each element will depend on its specific electronegativity and electron configuration.
Is Helium Positively or Negatively Charged?
Helium is a neutral atom, meaning it has no net charge. It consists of two positively charged protons and two negatively charged electrons, which cancel each other out to give a net charge of zero. So, helium is neither positively nor negatively charged. It is important to note that the charge of an atom is determined by the number of protons and electrons it has, and since these numbers are equal in a neutral atom, its overall charge is zero.
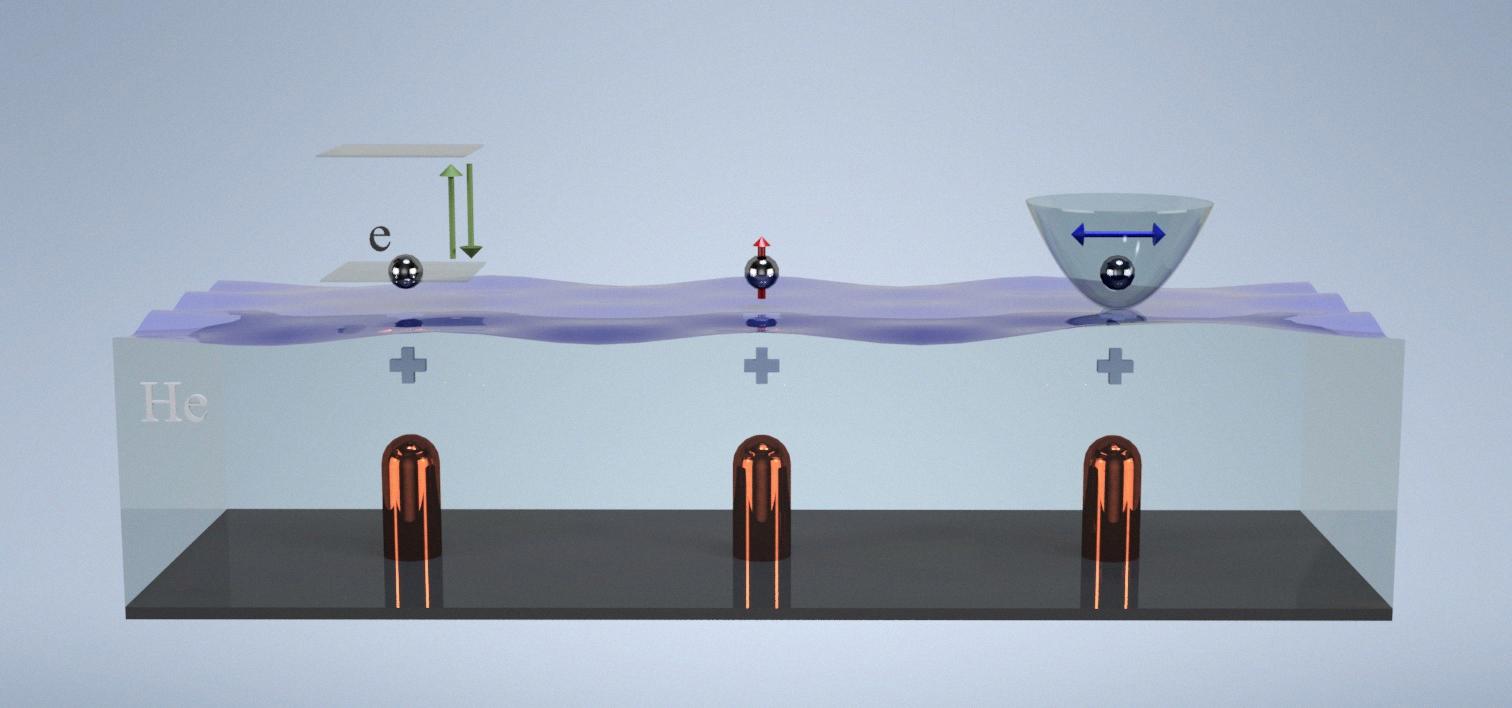
Source: en.wikipedia.org
Losing Electrons: An Overview
Metals are the elements that can lose electrons. This is because metals usually have few electrons in their outermost energy level, also known as the valence shell. In order to achieve a stable electron configuration, metals tend to lose one or more electrons from their valence shell to form positively charged ions, also known as cations. This process is called ionization or oxidation. The ease with which an element loses electrons is known as its electronegativity. Metals generally have a low electronegativity, making them good conductors of electricity and heat. Examples of metals include copper, iron, gold, and silver.
Why Helium Does Not Gain Electrons
Helium does not tend to gain electrons becuse it has a filled electron shell. The lowest electron shell of an atom can hold a maximum of 2 electrons, and helium has only 2 electrons in total. Therefore, its electron shell is already filled, and it does not have any room for additional electrons. Atoms with filled electron shells have a stable configuration, which means they do not need to lose, gain, or share electrons to achieve stability. As a result, helium does not become involved in chemical bonding with other atoms. Instead, it remains inert and does not react with other elements under normal conditions.
Elements That Do Not Gain or Lose Electrons
Noble Gases, also known as Group 18 elements, are known for their inertness and lack of reactivity. They do not tend to gain or lose electrons because they already have a full valence shell, which makes them stable and unreactive. The full valence shell of noble gases is due to their electronic configuration, which consists of a completely filled outermost shell. This electronic configuration makes it difficult for noble gases to bond with other elements and form compounds. In contrast, most other elements have incomplete valence shells and therefore tend to gain or lose electrons to achieve a full valence shell and beome stable.
Why Helium Does Not Form an Ion
Helium is a chemical element with atomic number 2 and is located in the group 18 of the periodic table. It is the second lightest element in the periodic table after hydrogen. Helium is a noble gas that is known for its low reactivity due to its completely filled outermost electron shell. The outermost shell of helium contains two electrons, which is the maximum number of electrons that can occupy that shell. This means that helium has no available electrons to lose or gain to form an ion.
When an atom loses or gains electrons, it becomes an ion with a positive or negative charge, respectively. The process of ionization requires energy to remove or add electrons to the atom. Helium has the highest ionization energy of all the elements, which means that it requires a lot of energy to remove an electron from helium. This makes it very difficult for helium to form an ion, as it would require an enormous amount of energy to remove an electron from its already stable electron shell.
Helium does not form an ion bcause it has a completely filled outermost electron shell with no available electrons to lose or gain. Additionally, it has a very high ionization energy, which makes it difficult to remove an electron from its stable configuration.
Source: commons.wikimedia.org
Is Helium a Neutral Atom or Ion?
Helium is a neutral atom. It consists of two positively charged protons and two negatively charged electrons. The electrons are located in an energy level outside of the nucleus, and their negative charges balance out the positive charges of the protons, resulting in a net charge of zero. If a helium atom were to lose or gain an electron, it would become a helium ion, which would carry a net positive or negative charge. However, in its natural state, helium is a neutral atom.
Does Helium Violate the Octet Rule?
The octet rule is a principle in chemistry that states that atoms tend to form compounds in ways that give them eight valence electrons, achieving the electron configuration of a noble gas. However, helium is an exception to this rule because it only has two valence electrons in its outermost energy level. Thus, helium does not break the octet rule because it only has two valence electrons and does not aim to achieve an octet configuration like oter elements. Helium is a noble gas, and its electron configuration is already stable, making it a very unreactive element that rarely forms chemical bonds with other elements.
Why is Helium Considered a Noble Gas?
Helium is a noble gas because of its electronic configuration. Helium has two electrons in its outermost shell, which is also called the valence shell. This valence shell is completely filled with electrons, making helium very stable and non-reactive toards other elements. Since helium does not need to accept or donate electrons to achieve a stable configuration, it does not form chemical bonds with other elements. This inertness and unwillingness to react with other elements is what makes helium a noble gas. In addition, all of the other noble gases, such as neon, argon, krypton, xenon, and radon, also have completely filled valence shells, which makes them stable and non-reactive. This characteristic is very valuable in various applications, such as in balloons, gas lasers, and welding, where the inertness of helium prevents unwanted reactions with other substances.
Gaining Electrons: Atoms That Participate
Atoms that have nearly eight electrons in teir valence shell tend to gain additional electrons to achieve a stable octet configuration, which is a stable electron configuration with eight valence electrons. These atoms are typically located on the right side of the periodic table, including elements such as oxygen, sulfur, and chlorine. When these atoms gain electrons, they become negatively charged ions known as anions. The process of gaining electrons is called reduction, and it occurs when an atom or ion accepts one or more electrons. The ability of an atom to gain electrons depends on its electronegativity, which is a measure of its attraction for electrons. Atoms with high electronegativity tend to gain electrons more readily than those with low electronegativity.
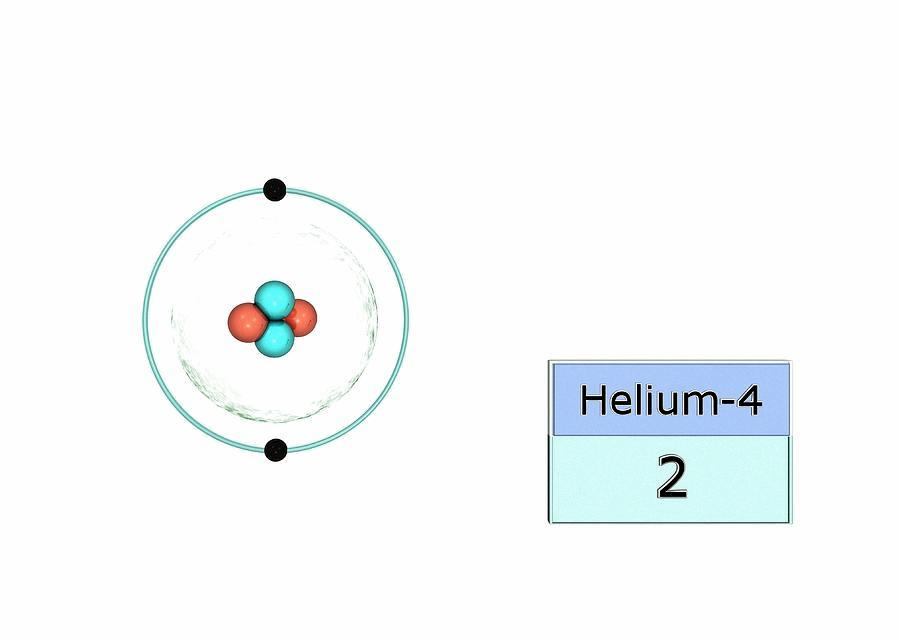
Source: fineartamerica.com
Elements That Gain Electrons
Nonmetals are the elements that tend to gain electrons. Nonmetals are located on the right side of the periodic table and have high electronegativity values. These elements have an outermost electron shell that is almost complete, and they only need to gain a few electrons to achieve a stable electron configuration. Nonmetals tend to form negative ions by gaining one or more electrons. This is due to the fact that nonmetals have a higher affinity for electrons than metals. By gaining electrons, nonmetals are able to achieve a more stable electron configuration and become more chemically stable. Examples of nonmetals that tend to gain electrons include oxygen, fluorine, chlorine, nitrogen, and sulfur.
Conclusion
Helium is a noble gas located in Group 18 of the periodic table. It has two electrons in an energy level outside the nucleus, making it stable and unlikely to gain or lose electrons. Helium does not share electrons with other atoms nor react with other elements. Due to the complete shell of electrons, the atom does not readily accept any extra electrons nor join with anything to make covalent compounds. Helium is widely used in various industries, including medical imaging, welding, and as a coolant for nuclear reactors. Its unique properties make it a valuable resource for many applications.
