Conductivity is one of the most important properties of a material that determines its ability to conduct electricity. It is defined as the measure of a material’s ability to conduct an electric current. Conductivity is a crucial factor in various fields of science, from materials science to chemistry, and plays a significant role in many industrial applications.
One of the most common questions about conductivity is whther it increases with temperature. The answer is not as straightforward as one might think. In some materials, conductivity increases with temperature, while in others, it decreases. Let’s take a closer look at the relationship between temperature and conductivity.
In metals, an increase in temperature results in an increase in resistance and a decrease in conductivity. This is because when the temperature of a metal increases, the metal ions vibrate more vigorously. This increased vibration makes it harder for the electrons to move through the metal, thereby increasing the resistance and decreasing the conductivity. This phenomenon is known as the temperature coefficient of resistance.
On the other hand, in some materials such as semiconductors, the conductivity increases with temperature. This is because as the temperature of a semiconductor increases, more electrons are excited from the valence band to the conduction band, thereby increasing the number of free electrons available to conduct electricity. This phenomenon is known as intrinsic conduction.
In liquids, the relationship between temperature and conductivity is more straightforward. As the temperature of a liquid increases, the movement of ions in the liquid also increases, resulting in an increase in conductivity. This is because ions in a liquid move more freely at higher temperatures, making it easier for an electric current to pass through the liquid.
The relationship between temperature and conductivity depends on the material being considered. In metals, an increase in temperature leads to an increase in resistance and a decrease in conductivity, while in some materials such as semiconductors, the conductivity increases with temperature. In liquids, an increase in temperature leads to an increase in conductivity. Understanding the relationship between temperature and conductivity is crucial for predicting the behavior of materials in various applications and designing new materials with specific conductivity properties.
The Impact of Temperature on Conductivity
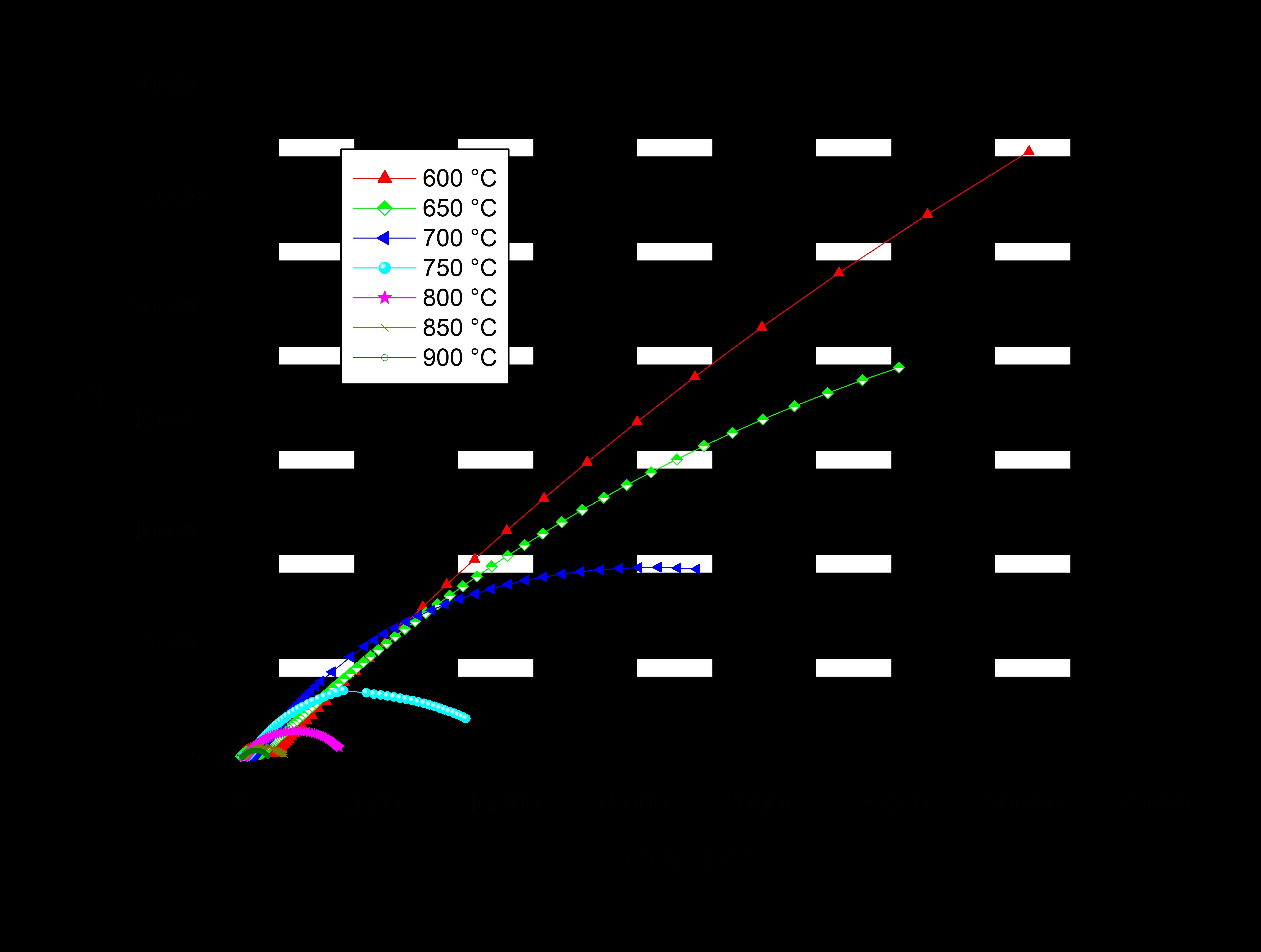
Temperature has a significant effect on the conductivity of a material. As the temperature of a material increases, the vibration of its metal ions also increases. This increase in vibration causes more collisions between the metal ions and the electrons, which results in a decrease in conductivity.
This decrease in conductivity occurs because as the metal ions vibrate more, they become more likely to collide with the electrons that are carrying an electrical charge through the material. These collisions can cause the electrons to lose some of thir energy, which slows them down and reduces the flow of electricity through the material.
Furthermore, as the temperature of a material increases, some of the electrons within it may gain enough energy to break free from their atoms and become free electrons. These free electrons can then collide with other electrons and metal ions, causing further disruption to the flow of electricity and a decrease in conductivity.
A higher temperature increases the vibration of metal ions, which leads to more collisions between the metal ions and the electrons and a decrease in conductivity.
Source: biologic.net
Effect of Temperature on Metal Conductivity
Metal conductivity does not increase with temperature. In fact, the opposite happens: as the temperature of a metal conductor increases, its conductivity decreases. This is beause when the temperature of a metal increases, the positive ions inside the metal conductor begin to vibrate more, which in turn leads to an increase in the thermal speed of the electrons. This increase in thermal speed makes it more difficult for the electrons to move through the metal, resulting in an increase in resistance and a decrease in conductivity. while metals are generally good conductors of electricity, their conductivity decreases as the temperature increases.
The Effects of Increased Conductivity
Conductivity can increase due to a variety of factors. One of the main contributors to increased conductivity is rising water temperatures. As water temperatures increase, the movement and energy of ions and molecules within the water also increase, leading to an increase in electrical conductivity. Additionally, if a lake or body of water does not receive enough fresh water from rain or streams, the concentration of dissolved salts in the water can increase, leading to an increase in conductivity. This is because whie water evaporates from the lake, the salts remain behind, leading to an increase in their overall concentration. conductivity can increase due to rising water temperatures and/or a decrease in the amount of fresh water entering a body of water.
Effect of Temperature on Conductivity
The effect of temperature on conductivity depends on the nature of the conductor. In metals, as the temperature increases, the vibration of metal ions increases, which results in an increase in resistance and a decrease in conductivity. This is becase the increased vibration of the ions leads to more collisions between electrons and ions, which hinders the flow of electrons, and hence, reduces conductivity.
On the other hand, in electrolytic conductors, the ions are the charge carriers, and with an increase in temperature, the ionization increases, resulting in an increase in conductivity. This is because the increased temperature provides more energy to the ions, which leads to the breaking of the ionic bonds and the release of more charge carriers, thus increasing the conductivity.
Therefore, the effect of temperature on conductivity is dependent on the type of conductor. While temperature decrease conductivity in metals, it increases conductivity in electrolytic conductors.
Effect of Conductivity on a System
The conductivity of water increases as salinity increases. This is because dissolved salts and other inorganic chemicals conduct electrical current, and as the amount of dissolved salts in water increases, so does its ability to pass an electrical current. Therefore, when the salinity level of water increases, it will lead to an increase in its conductivity. Conversely, a decrease in salinity will result in a decrease in conductivity. It is important to note that while salinity is the primary factor affecting conductivity, other factors such as temperature and the presence of organic matter can also influence conductivity levels.
Effect of Temperature on Conductivity
The answer to this question depends on the type of material we are considering. In general, for metals, conductivity decreases with an increase in temperature. This is because metals have free electrons that are responsible for the flow of electricity. When the temperature increases, the free electrons collide more frequently with the atoms and ions in the metal, which results in increased resistance and decreased conductivity.
On the other hand, for non-metals, conductivity increases with an increase in temperature. This is because non-metals do not have free electrons, and only the molecular vibrations are responsible for conduction of heat. As the temperature increases, the molecular vibrations become more energetic, resulting in increased thermal conductivity.
It is important to note that there can be exceptions to thee general trends, and the behavior of a specific material may depend on its composition, crystal structure, and other factors. Thus, it is crucial to study the conductivity-temperature relationship on a case-by-case basis to fully understand the behavior of a material.
Factors Influencing Electrical Conductivity
Conductivity, the ability of a solution to conduct electricity, is influenced by several factors. The concentration of ions in a solution is one of the most significant factors affecting conductivity. As the concentration of ions in a solution increases, the number of charged particles that can carry an electric current also increases, resulting in higher conductivity.
The type of ions present in a solution also affects its conductivity. Ions with higher charge densities and smaller sizes can move more easily through a solution and conduct electricity more efficiently. For example, solutions containing monovalent ions, such as Na+ and Cl-, have higher conductivity than solutions containing divalent ions, such as Ca2+ and Mg2+.
Temperature has a considerable impact on the conductivity of a solution. As the temperature increases, the kinetic energy of the ions in the solution also increases. This leads to a higher rate of ion movement, resulting in higher conductivity. However, at extremely high temperatures, the conductivity may decrease because the ions becme too agitated, leading to increased resistance.
The concentration of ions, the type of ions present, and the temperature of the solution are the three main factors affecting conductivity, and understanding these factors is crucial in many fields, including chemistry, biology, and engineering.
The Impact of Temperature on Copper Conductivity
Copper conductivity does not increase with temperature. In fact, the opposite occurs – copper conductivity decreases as temperature increases. This is due to the fact that as temperature increases, the atoms in the copper lattice vibrate more rapidly, leading to increased collisions between electrons and lattice atoms. These collisions scatter the electrons, leading to an overall decrease in conductivity. Additionally, copper has a positive temperature coefficient, meaning that its resistance increases with temperature. Therefore, the conductivity of copper decreases as temperature increases due to both increased electron scattering and increased resistance.
The Effect of pH on Conductivity
PH does affect conductivity. pH is a measure of the concentration of hydrogen ions in a solution. The higher the concentration of hydrogen ions, the lower the pH value, and the greater the conductivity of the solution. This is beause the presence of more ions in the solution allows for more charge to be carried through the solution, thus increasing conductivity. Conversely, a decrease in pH, or an increase in the concentration of hydroxide ions, will decrease conductivity. It’s important to note that pH is not the only factor that affects conductivity, as the concentration and mobility of ions in a solution also play a role.
The Impact of Salt on Conductivity
Salt is composed of charged particles called ions, which dissociate into positively charged cations and negatively charged anions when dissolved in water. These ions are free to move about in the water, and when an electric field is applied, they can carry electrical current through the solution. This is because the movement of ions allos for the flow of electrons, which is what constitutes an electric current. Therefore, the more salt that is dissolved in the water, the more ions there are available to carry electrical current, resulting in an increase in conductivity. the presence of dissolved ions in saltwater allows for the flow of electrical current, which is why salt increases conductivity.
Conductivity of Water
Water conductivity is a measure of how easily an electric current can pass through it. It is typically measured in microsiemens per centimeter (µS/cm) or millisiemens per centimeter (mS/cm). The conductivity of water varies depending on its source and the presence of dissolved minerals and salts. Pure distilled and deionized water, which contains no dissolved substances, has a very low conductivity of 0.05 µS/cm. Seawater, on the othr hand, has a high conductivity of around 50 mS/cm due to its high salt content. Drinking water typically has a conductivity range of 200 to 800 µS/cm, depending on the amount of dissolved minerals and salts present. Rain or snow water has a conductivity range of 2 to 100 µS/cm, which is also affected by the presence of dissolved substances in the atmosphere. water conductivity is an important factor in determining its suitability for various uses, such as drinking, agriculture, and industrial processes.
Conclusion
Conductivity is an important property of materials that determines their ability to conduct electric current. It is influenced by severl factors, including temperature, concentration of ions or particles in the solution, and the nature of the solvent. Metals are good conductors of electricity due to the presence of free electrons, but their conductivity decreases as their temperature increases. In contrast, the conductivity of solutions increases with temperature due to increased ion mobility. The salinity of water also affects its conductivity, and high salinity levels may indicate contamination or drought conditions. Understanding conductivity is essential for many scientific and industrial applications, from designing electronic devices to monitoring water quality in natural ecosystems.
