Carbon tetrachloride, also known as CCl4, is a colorless and heavy liquid with a sweet odor. It is widely used as a solvent for oils, fats, and waxes, and also as a fire extinguisher. CCl4 is an interesting molecule to study because it has unique properties that make it different from oter molecules. One of the questions that often comes up is whether or not CCl4 has dipole-dipole forces.
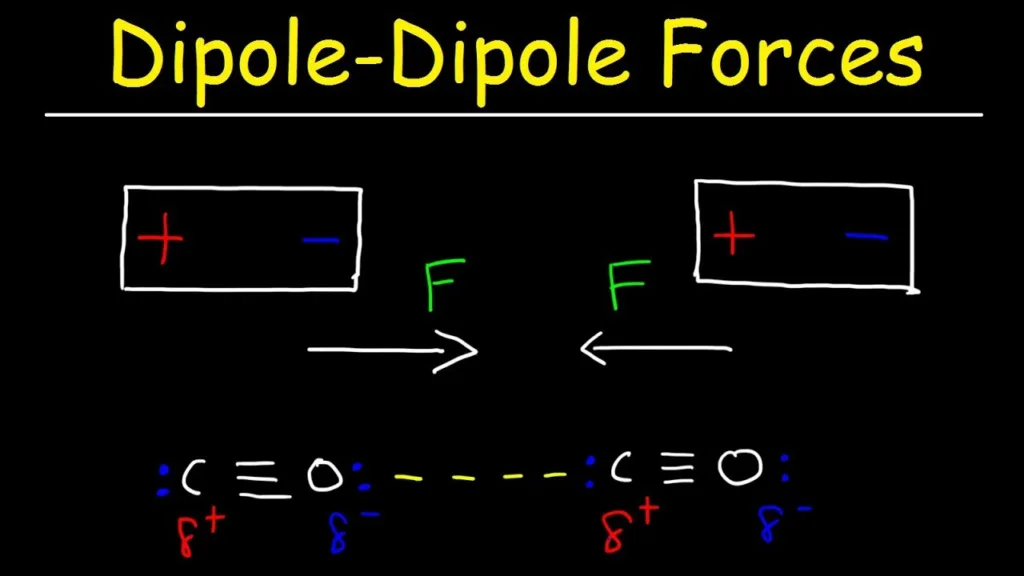
Dipole-dipole forces are intermolecular forces that exist between polar molecules. These forces arise due to the positive and negative charges on the molecules. When two polar molecules come close together, the positive end of one molecule is attracted to the negative end of the other molecule, and vice versa. This attraction leads to the formation of a dipole-dipole interaction.
However, CCl4 is a nonpolar molecule. It has four chlorine atoms arranged symmetrically around a central carbon atom. The electronegativity of chlorine is higher than that of carbon, which means that the electrons are drawn more towards the chlorine atoms. As a result, there is a slight negative charge around each chlorine atom and a slight positive charge around the carbon atom. However, these charges are distributed symmetrically around the molecule, which means that the molecule has no net dipole moment.
This lack of a net dipole moment means that CCl4 does not have dipole-dipole forces. Instead, the strongest intermolecular force between CCl4 molecules is London dispersion forces, also known as induced-dipole forces. These forces arise due to the temporary shifts in the electron distribution around the molecule, leading to the formation of a temporary dipole. This temporary dipole can then induce a temporary dipole in a neighboring molecule, leading to an attractive force between the two molecules.
CCl4 is a nonpolar molecule that does not have dipole-dipole forces. Instead, the strongest intermolecular force between CCl4 molecules is London dispersion forces. This unique property of CCl4 makes it an important molecule to study, and it has many practical applications in industry and research.
Interaction Type of CCl4
CCl4 is a commonly used chemical compound that is utilized in various applications. When it comes to its bonding nature, CCl4 is a non-polar molecule with a tetrahedral shape. In terms of intermolecular forces, the bonds between CCl4 are primarily dispersion or London forces, which are also known as induced-dipole forces. This is because CCl4 does not have a net dipole moment due to the symmetry of its four C-Cl bonds, meaning that the molecule has no overall positive or negative charge distribution. Therefore, the attractive forces between individual CCl4 molecules are due to the temporary induced dipoles that arise from the random fluctuations of electron density withn the molecule. As a result, CCl4 is considered to have weak intermolecular forces and a low boiling point.
Source: learningchemistryeasily.blogspot.com
Intermolecular Forces Found in CCl4
CCl4, also known as carbon tetrachloride, is a tetrahedral molecule with four identical polar C-Cl bonds. However, due to its symmetrical tetrahedral geometry, the overall dipole moment of the molecule is zero, making it nonpolar.
The intermolecular forces found in CCl4 are London dispersion forces, which are the weakest type of intermolecular forces. These forces occur due to temporary fluctuations in the electron density of the molecule, creating instantaneous dipoles that can induce a dipole in an adjacent molecule.
London dispersion forces increase with the size and surface area of the molecule, and CCl4 has a relatively large surface area, allowing for stronger dispersion forces than smaller molecules.
CCl4 is a nonpolar molecule with its strongest intermolecular forces bing London dispersion forces.
Does CCl4 Have Dipole Moments?
CCl4 does not have dipoles. This is because the molecule has a tetrahedral geometry, with the four chlorine atoms arranged symmetrically around the carbon atom. Each chlorine atom has a higher electronegativity than carbon, meaning that the shared electrons in the carbon-chlorine bonds are pulled more towards the chlorine atoms. However, since the four chlorine atoms are arranged symmetrically, the bond dipoles cancel out each other, resulting in a net dipole moment of zero. Therefore, CCl4 is a nonpolar molecule.
Dipole-Dipole Forces in CH4
CH4 does not have dipole-dipole forces. This is because it is a nonpolar molecule, meaning that the electronegativity difference between the carbon and hydrogen atoms is very small, resulting in no significant charge separation within the molecule. Dipole-dipole forces occur between polar molecules where there is a significant difference in electronegativity among the atoms, causing partial charges on the molecule. Instead, CH4 has London dispersion forces, which are weak intermolecular attractions that arise due to the temporary fluctuations in electron density within the molecule.
Absence of Dipole in CCl4
Carbon tetrachloride (CCl4) is a chemical compound composed of one carbon atom and four chlorine atoms. The molecule has a tetrahedral shape, with the carbon atom at its center and the four chlorine atoms positioned at the four vertices of the tetrahedron.
The reason why carbon tetrachloride has no dipole moment is due to its symmetrical shape. The four C-Cl bonds in the molecule are identical and are oriented in a tetrahedral arrangement, with the bond angles between them beng 109.5 degrees. Each bond has a dipole moment that points toward the electronegative chlorine atom, resulting in a net dipole moment of zero.
Moreover, the electronegativity of carbon and chlorine atoms is not significantly different, so the electron density is distributed evenly across the molecule. This results in no separation of charges, hence no dipole moment.
The symmetrical arrangement of the four C-Cl bonds in carbon tetrachloride and the even distribution of electron density across the molecule result in no net dipole moment.
Absence of Dipole Moment in CCl4
Carbon tetrafluoride (CCl4) is a molecule composed of one carbon atom and four chlorine atoms arranged in a tetrahedral shape. Each of the four chlorine atoms is bonded to the carbon atom trough a covalent bond, and each of these bonds is polar due to the difference in electronegativity between chlorine and carbon.
However, the tetrahedral shape of CCl4 ensures that the polarities of these bonds cancel each other out, resulting in a molecule with no net dipole moment. This is because the polar bonds are oriented towards the four corners of the tetrahedron, and their vector sum is zero.
In other words, the partial positive charges on the carbon atom are balanced by the partial negative charges on the four chlorine atoms, resulting in a symmetrical distribution of charges throughout the molecule. As a result, CCl4 is a nonpolar molecule with no dipole moment.
Polarity of CCl4 Dipole
Carbon tetrachloride (CCl4) is a nonpolar compound. A molecule is said to be polar if its centers of positive and negative charges do not coincide, resulting in a net dipole moment. However, in the case of CCl4, the chlorine atoms are arranged symmetrically around the central carbon atom, forming a tetrahedral geometry. As a result, the bond dipoles of the four C-Cl bonds cancel each other, resulting in a net dipole moment of zero. Hence, CCl4 does not exhibit any polarity, making it a nonpolar molecule.
Does CCl4 Have a Significant Dipole Moment?
Carbon tetrachloride (CCl4) is a tetrahedral molecule with four identical C-Cl bonds formed by the sharing of electrons between the carbon and chlorine atoms. The electronegativity of chlorine is higher than that of carbon, resulting in a partial negative charge on the chlorine atoms and a partial positive charge on the carbon atom.
However, in CCl4, the four C-Cl bonds are arranged symmetrically aound the central carbon atom, resulting in a cancelation of the dipole moments of each bond. Therefore, the net dipole moment of the molecule is zero, making it a nonpolar molecule.
CCl4 does not have a large dipole moment due to its symmetrical molecular structure that causes the dipole moments of each bond to cancel out.
The Nature of London Dispersion Forces in CCl4
CCl4 exhibits London dispersion force as it is a nonpolar molecule. London dispersion force is an intermolecular force that occurs between all molecules, whether they are polar or nonpolar. However, it is the only intermolecular force that exists between nonpolar molecules. The London dispersion force arises due to the temporary dipoles that occur within the molecule as a result of the uneven distribution of electrons. These temporary dipoles cause the electrons in the neighboring molecules to repel or attract each other, leading to the attractive force between the molecules. Because CCl4 is a nonpolar molecule, it does not have permanent dipoles. However, it has temporary dipoles, and thee temporary dipoles are responsible for the London dispersion force in CCl4.
Molecules with Dipole-Dipole Forces
Molecules that have permanent dipoles, such as polar molecules, exhibit dipole-dipole forces. These forces arise due to the attraction between the positive and negative ends of the dipoles. The strength of these forces increases with increasing polarity of the molecule. In addition, polar molecules can induce dipoles in nonpolar molecules, resulting in dipole-induced dipole forces. Therefore, any molecule with a permanent dipole moment, either due to differences in electronegativity or molecular geometry, will exhibit dipole-dipole forces. Examples of such polar molecules include water (H2O), ammonia (NH3), hydrogen fluoride (HF), and hydrogen sulfide (H2S).
Examples of Dipole-Dipole Forces
Dipole-dipole forces are intermolecular attractions that occur between polar molecules due to the partial positive and partial negative charges on teir atoms. Examples of dipole-dipole forces include hydrogen chloride (HCl), hydrogen fluoride (HF), and water (H2O). HCl is a permanent dipole due to the difference in electronegativity between hydrogen and chlorine, resulting in a partial positive charge on hydrogen and a partial negative charge on chlorine. HF also has a permanent dipole due to the difference in electronegativity between hydrogen and fluorine. Water, on the other hand, has a bent shape and a polar covalent bond between oxygen and hydrogen, resulting in a partial negative charge on oxygen and partial positive charges on hydrogen atoms. These partial charges attract neighboring water molecules, resulting in dipole-dipole forces. dipole-dipole forces play a crucial role in determining the physical and chemical properties of polar molecules.
Number of Dipoles in CCl4
CCl4, or carbon tetrachloride, has four polar bonds. Each bond between carbon and chlorine is polar due to the difference in their electronegativities. This results in an unequal sharing of electrons, with the chlorine atom pulling the shared electrons closer to itself and developing a partial negative charge, while the carbon atom develops a partial positive charge. As a result, each carbon-chlorine bond has a dipole moment. However, despite having four polar bonds, the molecule of CCl4 is nonpolar becase of its symmetrical tetrahedral geometry. The dipole moments of individual bonds cancel out each other, resulting in a net zero dipole moment for the molecule.
Does NH3 Have Dipole-Dipole Forces?
NH₃ (ammonia) has dipole-dipole forces. Dipole-dipole forces are a type of intermolecular force that occurs between polar molecules. NH₃ is a polar molecule because of the electronegativity difference between nitrogen and hydrogen atoms. Nitrogen is more electronegative than hydrogen, which cuses a partial negative charge to develop on the nitrogen atom and a partial positive charge on the hydrogen atoms. This results in a dipole moment in NH₃, making it a polar molecule. The polar nature of NH₃ allows it to have dipole-dipole forces with other polar molecules. These forces are weaker than hydrogen bonding, but stronger than dispersion forces.
Does Carbon Dioxide Have Dipole-Dipole Forces?
Carbon dioxide (CO2) is a linear molecule consisting of two oxygen atoms and one carbon atom. The molecule has a polar covalent bond due to the difference in electronegativity between the carbon and oxygen atoms. However, despite having polar bonds, carbon dioxide does not have dipole-dipole forces. This is because the molecule is symmetric, and the dipole moments of the two polar bonds cancel each other out. Therefore, the net dipole moment of the molecule is zero, making it a nonpolar molecule. Consequently, carbon dioxide does not exhibit dipole-dipole forces.
Does Chlorine (Cl2) Have Dipole-Dipole Forces?
Cl2 does not have dipole-dipole forces. Dipole-dipole forces arise due to the presence of a permanent dipole moment in a molecule. However, Cl2 is a nonpolar molecule because the two atoms of chlorine have equal electronegativity, and the bond between them is a nonpolar covalent bond. As a result, the electron density is evenly distributed in the molecule, and no permanent dipole moment exists. Therefore, Cl2 cannot exhibit dipole-dipole forces. Instead, it exhibits weak van der Waals forces, which arise due to temporary fluctuations in the electron density of the molecule.
Conclusion
CCl4 or carbon tetrachloride is a nonpolar molecule with a tetrahedral shape. It is composed of four chlorine atoms covalently bonded to a single carbon atom. Due to its symmetrical structure, the molecule has zero dipole moment, which means that it has no positive or negative poles. As a result, CCl4 only exhibits London dispersion forces, which are the weakest intermolecular forces. These forces arise from temporary fluctuations in electron density that cause the formation of an induced dipole in neighboring molecules. Despite its weak intermolecular forces, CCl4 is commonly used as a solvent, a refrigerant, and in fire extinguishers. However, it is also highly toxic and carcinogenic, which has led to its restricted use in many countries.
