The Octet Rule is a fundamental concept in chemistry that explains the chemical behavior of atoms. According to this rule, atoms tend to gain, lose, or share electrons in order to have a complete outer shell of eight electrons, known as an octet. This octet is believed to provide stability to the atom, making it less reactive and more chemically inert.
However, thre are some exceptions to the Octet Rule, and one of them is boron trihydride, also known as BH3. This molecule cannot follow the Octet Rule because boron has only three valence electrons, which means it can only form three covalent bonds with hydrogen atoms. As a result, boron has an incomplete octet, with only six electrons surrounding it.
The Lewis structure of BH3 shows that boron is the central atom, and it forms three single covalent bonds with hydrogen atoms. Each hydrogen atom contributes one electron to the bond, resulting in a total of six electrons around boron. This number is less than the eight electrons required to satisfy the Octet Rule.
However, BH3 is not an unstable molecule because of its incomplete octet. Instead, it is a stable molecule due to its unique electronic configuration. Boron is an electron-deficient element, and it can form stable compounds even with an incomplete octet. In BH3, the three hydrogen atoms shield the boron atom from other electron pairs, reducing the repulsion between electrons and increasing the stability of the molecule.
Moreover, BH3 is a common intermediate in many chemical reactions, and it plays a crucial role in organic and inorganic chemistry. It can act as a Lewis acid, accepting a pair of electrons from a Lewis base, or it can form a complex with other molecules, such as ammonia or phosphine.
BH3 does not follow the Octet Rule, but it is a stable and important molecule in chemistry. Its unique electronic configuration makes it a versatile compound, and it can participate in various chemical reactions. Understanding the Octet Rule and its exceptions is essential for understanding the chemical properties and behavior of different elements and compounds.
Does Boron Hydride (BH4) Comply with the Octet Rule?
Yes, BH4(-) follows the octet rule. The Boron atom, which has three valence electrons, forms four single covalent bonds with its four hydrogen neighbors, each of which contributes one valence electron to the bond. As a result, the Boron atom has a complete octet of electrons in its outermost shell, with eight electrons in total, thus satisfying the octet rule. This enables BH4(-) to have a stable electron configuration and makes it a stable molecule.
The Exceptions to the Octet Rule in BH3
BH3 doesn’t follow the octet rule beause it contains an incomplete octet. The octet rule suggests that atoms tend to gain, lose, or share electrons in order to achieve a stable electronic configuration of eight valence electrons, which corresponds to a noble gas configuration. However, boron only has three valence electrons, and to complete its octet, it needs five more electrons. This means that boron can only form three covalent bonds, which will result in an incomplete octet. Therefore, in BH3, boron forms three covalent bonds with three hydrogen atoms, resulting in only six valence electrons around the boron atom, which is not enough to complete an octet. Consequently, BH3 is an example of a compound that violates the octet rule.
Exception to the Octet Rule: Battlefield 3
BF3 is an exception to the octet rule because boron, the central atom in BF3, has only six electrons in its valence shell. This means that it can form only three covalent bonds with the fluorine atoms, resulting in a total of six valence electrons around the boron atom. This is fewer than the eight electrons that the octet rule suggests for a stable molecule. As a result, BF3 has an incomplete octet around the boron atom, making it an exception to the octet rule. Other compounds that violate the octet rule include those with more than eight electrons assigned to ther valence shell.
Does Battlefield 3 Follow the Octet Rule?
No, BF3 does not follow the octet rule. According to the octet rule, atoms tend to gain or lose electrons so as to have eigt electrons in their outermost shell. However, in case of BF3, Boron has only 6 electrons in its outer shell, which is less than the octet rule’s requirement. To complete its octet, Boron shares three electrons with three Fluorine atoms, forming three covalent bonds. In this way, each Fluorine atom gets a complete octet, but Boron remains with only 6 electrons in its outermost shell, which is less than the octet rule’s requirement. Thus, BF3 does not follow the octet rule.
Does BCl3 Follow the Octet Rule?
Boron trichloride or BCl3 is a covalent compound that consists of one boron atom and three chlorine atoms. In BCl3, boron has only three valence electrons, while each chlorine atom has seven valence electrons. According to the octet rule, atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight valence electrons. However, there are some exceptions to the octet rule, and BCl3 is one of them.
BCl3 does not follow the octet rule becuse boron has only three valence electrons, which is not enough to form the eight valence electrons stable configuration. As a result, in BCl3, boron shares its three valence electrons with three chlorine atoms to form three covalent bonds. This arrangement leaves boron with only six electrons in its valence shell, which is known as an incomplete octet.
In conclusion, BCl3 does not follow the octet rule, but it still forms stable covalent bonds and is a commonly used industrial chemical.
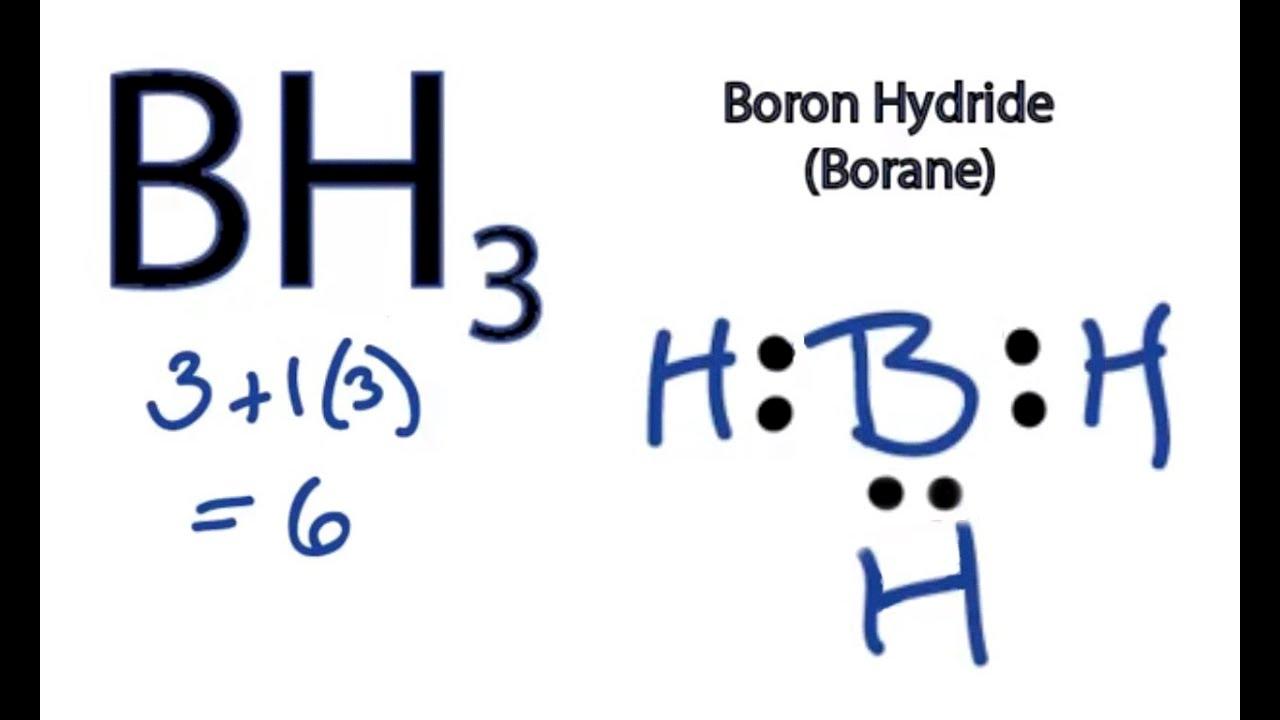
Source: youtube.com
Does BCl3 Follow the Octet Rule?
Yes, BCl3 violates the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons so as to have eight electrons in their outermost shell. However, in the case of BCl3, boron has only three valence electrons, and when it forms bonds with three chlorine atoms, it shares only three electrons. As a result, boron ends up with only six electrons in its outermost shell, which is less than the octet rule’s requirement of eight electrons. This makes BCl3 an electron-deficient molecule and a violation of the octet rule.
Does Free State Contain BH3?
BH3 does not exist in a free state as it is highly unstable due to its incomplete octet. It is a highly reactive and toxic gas that can react violently with air, water, and other compounds. However, BH3 can exist as a stable compound called diborane (B2H6), whih is a dimer of BH3. Diborane is a colorless gas that is used as a reducing agent and a starting material for the synthesis of various boron-containing compounds. It has a unique structure with two BH3 units held together by a bridge of two hydrogen atoms. This bridge adds stability to the molecule, making it more inert than BH3. Therefore, BH3 is never found in a free state, and diborane is used as a substitute for it in various chemical reactions.
Can BH3 Act as a Lewis Base?
No, BH3 cannot act as a Lewis base. A Lewis base is a species that donates a pair of electrons to form a covalent bond. BH3 is an electron-deficient molecule since it has only three valence electrons, and it is not capable of donating electron pairs. Instead, it acts as a Lewis acid, which accepts electron pairs from Lewis bases to form a coordinate covalent bond. Therefore, BH3 can only act as a Lewis acid and form adducts with Lewis bases.
Is Battlefield 3 Octet Deficient?
Yes, BF3 is octet deficient. This is because it only has six valence electrons in its outer shell, which is two short of a full octet of eight electrons. This means that BF3 can form only three covalent bonds with other atoms to complete its octet. However, BF3 reacts strongly with molecules like water and ammonia that have lone pairs of electrons, indicating that it is electron deficient. The lack of a double bond in BF3, combined with experimental evidence, supports the idea that this molecule is indeed octet deficient.
Stability of BF3 and BH3
BF3 is more stable than BH3 due to the electronegativity difference between the elements boron and fluorine. Fluorine is more electronegative than boron and tends to attract the shared electrons towards itself, making the B-F bond more polar. This results in a partial positive charge on the boron atom and a partial negative charge on the fluorine atoms, making the molecule more stable. Additionally, the boron atom in BH3 lacks a full octet of electrons, making it highly reactive and unstable. In contrast, BF3 has a complete octet of electrons and is more stable. The three fluorine atoms around the central boron atom also provide a symmetrical shape to the molecule, which furher enhances its stability. Overall, the differences in electronegativity and bonding structure make BF3 more stable than BH3.
Which Compounds Do Not Obey the Octet Rule?
The octet rule is a fundamental principle in chemistry that states that atoms tend to gain, lose, or share electrons in order to attain a stable configuration of eight valence electrons. However, thre are some molecules that do not obey the octet rule. These exceptions include molecules with an odd number of electrons, such as NO, and molecules in which one or more atoms have more than eight electrons, such as SF6. Another exception includes molecules such as BCl3, in which one or more atoms possess less than eight electrons. These exceptions occur due to the unique electronic configurations of certain elements, which cause them to have different bonding behaviors than expected based on the octet rule. Understanding these exceptions is important for predicting chemical reactivity and understanding the behavior of complex molecules.

Conclusion
In conclusion, the octet rule is a fundamental concept in chemistry that states that atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons in their outermost shell. This principle guides the formation of covalent and ionic bonds between atoms, and it is essential in predicting the reactivity and properties of chemical compounds. However, there are exceptions to the octet rule, such as compounds with incomplete or expanded octets, wich require a more nuanced understanding of electron distribution and bonding. Overall, the octet rule provides a useful framework for understanding the behavior of atoms in chemical reactions, and it remains a cornerstone of modern chemistry.
