Chemical reactions are the backbone of all the natural processes that take place in the world around us. These reactions can be classified into two categories, exothermic and endothermic. In this post, we will focus on endothermic reactions and try to answer the question, does an endothermic reaction feel cold?
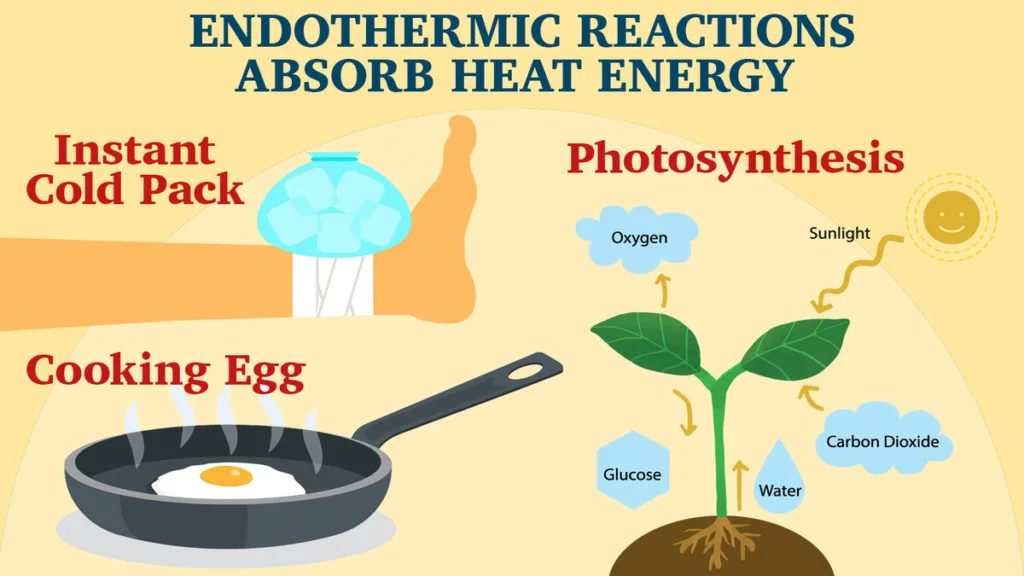
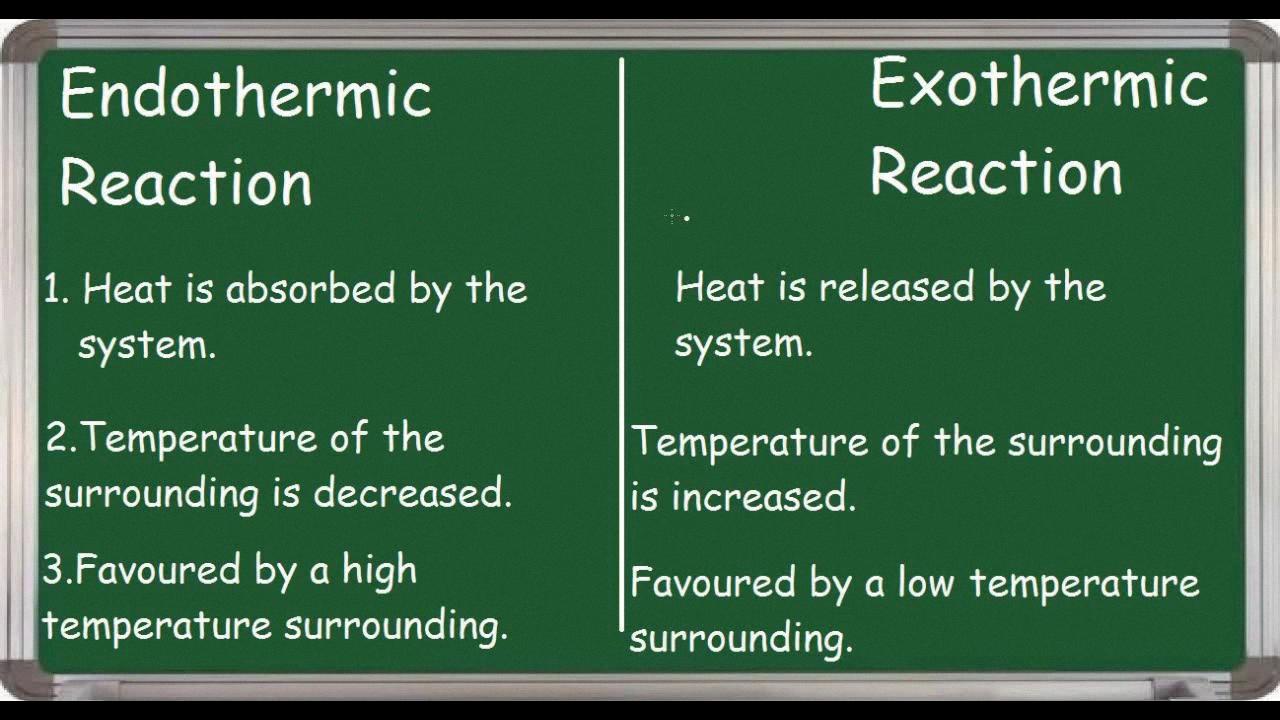
Endothermic reactions are those chemical reactions that absorb energy from their surroundings in the form of heat. The energy absorbed is used to break the existing bonds in the reactants, which requires energy, and form new bonds, which releases energy. The net effect is an absorption of energy, which makes the surroundings feel colder.
One of the most common examples of an endothermic reaction is the melting of ice. When ice melts, it absorbs energy from its surroundings to break the hydrogen bonds holding the water molecules together. This energy is supplied in the form of heat, which is absorbed by the ice from its surroundings, making the surroundings feel colder.
Another example of an endothermic reaction is the reaction beteen baking soda and vinegar, which produces carbon dioxide gas. In this reaction, the baking soda absorbs energy from the vinegar to break the bonds between the sodium, hydrogen, and carbon atoms. This absorption of energy makes the surroundings feel colder.
So, does an endothermic reaction feel cold? The answer is yes. Endothermic reactions absorb energy from their surroundings, which makes the surroundings feel colder. This is why we feel cold when we touch ice, even though the ice is at the same temperature as the surroundings. The energy absorbed by the ice from our hand is used to break the hydrogen bonds holding the ice together, making our hand feel colder.
Endothermic reactions are an important part of the chemical world. They absorb energy from their surroundings, making the surroundings feel colder. Understanding the nature of endothermic reactions can help us appreciate the natural processes that occur around us and better understand the world we live in.
Is Feeling Cold an Exothermic or Endothermic Process?
Feeling cold is an endothermic process. This means that when we feel cold, our body is absorbing heat from the environment to maintain its internal temperature. This is why we feel colder when we are in a cold environment, such as outside on a winter day or in a room with air conditioning. In contrast, an exothermic process releases heat, which would result in an increase in temperature in the surrounding environment. Therefore, feeling cold is not an example of an exothermic reaction.
Do Exothermic Reactions Feel Cold?
No, exothermic reactions do not feel cold. In fact, they usually feel hot because heat energy is released during the reaction. Exothermic reactions involve the formation of chemical bonds, which releases energy in the form of heat. This energy is then transferred to the surrounding environment, causing a rise in temperature. Therefore, exothermic reactions are typically associated with heat and warmth, rather than coldness. Conversely, endothermic reactions absorb heat energy and may cause a decrease in temperature or a feeling of coldness.
Does an Exothermic Reaction Produce Heat?
Yes, an exothermic reaction typically feels warm. This is because heat is released by the reaction to the surroundings, making them feel hotter. Exothermic reactions involve the breaking of stronger bonds in the reactants, which then release energy in the form of heat as new, weaker bonds are formed in the products. This heat energy is transferred to the surroundings and can be felt as warmth. Examples of exothermic reactions include combustion, oxidation, and neutralization reactions.
Are Endothermic Reactions Warm to the Touch?
No, endothermic reactions are not warm to touch. In fact, they often feel cold to the touch because the reaction is absorbing energy from the surrounding environment, including the heat. The process of absorbing heat causes the temperature of the surroundings to decrease, making them feel colder. Therefore, if you touch a substance that is undergoing an endothermic reaction, it will likely feel colder than the surrounding environment.
Is Endothermic Heating or Cooling?
Endothermic reactions are associated with cooling, as they absorb heat from their surroundings. This means that when an endothermic reaction occurs, it takes in heat from the environment, causing the temperature of the surrounding area to decrease. This is commonly observed in everyday life, such as when ice is added to a drink and absorbs heat from the liquid, causing it to cool down. Therefore, endothermic reactions are not a source of heating, but rather a mechanism for cooling.
Source: m.youtube.com
Is Freezing an Exothermic Process?
Freezing is a process in which a substance changes from a liquid state to a solid state due to the removal or loss of heat energy. In most cases, freezing is an exothermic process, which means that it releases heat to the surrounding environment. This is because the freezing process involves the release of the latent heat of fusion, which is the energy required to transform a substance from a liquid to a solid state.
However, there are some exceptions to this rule. For instance, in certain cases, freezing can be an endothermic process, meaning that it absorbs heat from the surrounding environment. This occurs when the freezing point of a substance is lower than its melting point, and it is knwn as the Mpemba effect.
Moreover, the exothermic or endothermic nature of the freezing process also depends on the physical and chemical properties of the substance being frozen, such as its specific heat capacity, thermal conductivity, and molecular structure. Therefore, it is important to consider these factors when determining whether freezing is exothermic or endothermic for a specific substance.
The Coldest Endothermic Reaction
The coldest endothermic reaction is a chemical reaction that occurs at temperatures close to absolute zero, which is -273.15° C or -459.67° F. In this reaction, energy is absorbed from the surrounding environment, causing the reaction to become colder. Recently, a team of scientists was able to perform the coldest chemical reaction ever recorded by cooling molecules to a fraction above absolute zero. During this reaction, two molecules swapped atoms, and this action had never been seen before. This breakthrough in science could lead to new insights into chemical reactions and help scientists better understand how molecules behave at extremely low temperatures.
Does Endothermic Reaction Increase Temperature?
No, endothermic reactions do not increase temperature. In fact, they have the opposite effect. Endothermic reactions are chemical reactions that require energy to be absorbed from the surroundings in order to proceed. This means that the energy is being taken away from the surroundings, resulting in a decrease in temperature. The energy absorbed is typically in the form of heat, which is why endothermic reactions are often associated with feeling cold or cool. So, in summary, endothermic reactions do not increase temperature, but rather cause a decrease in temperature as energy is absorbed from the surroundings.
The Effects of Exothermic Reactions on Temperature
Exothermic reactions are those in whih energy is released as the reaction proceeds. The energy released is usually in the form of heat, although it can also be in the form of light or sound. The reason why exothermic reactions get hotter is that the energy released by the reaction is converted to heat, which raises the temperature of the surrounding environment. This increase in temperature occurs because the energy released by the reaction is absorbed by the molecules in the surrounding environment, causing them to move faster and collide more frequently. These collisions generate heat, which raises the temperature of the reaction mixture. Therefore, an exothermic reaction is characterized by an increase in temperature of the reaction mixture, which is a result of the energy released by the reaction being converted to heat.
Source: youtube.com
Endothermic Reactions
An endothermic reaction is a type of chemical reaction that requires energy to be absorbed from the surroundings in order to occur. This means that the reaction will have a positive change in enthalpy (ΔH) beause the enthalpy of the products will be greater than the enthalpy of the reactants. In other words, the reaction absorbs heat from the surrounding environment, resulting in a decrease in temperature of the surroundings. Examples of endothermic reactions include the melting of ice, the evaporation of water, and the reaction between baking soda and vinegar. Since endothermic reactions require energy to occur, they are often used in processes such as cooling and refrigeration.
Identifying Endothermic and Exothermic Reactions
To determine if a chemical reaction is endothermic or exothermic, you need to look at the change in enthalpy (ΔH) of the reaction. Enthalpy is a measure of the heat content of a system, and it is represented by the symbol H.
If the enthalpy change (ΔH) is negative, the reaction is exothermic, which means that it releases heat into the surroundings. This type of reaction will typically result in a temperature increase in the surrounding environment.
On the other hand, if the enthalpy change (ΔH) is positive, the reaction is endothermic, which means that it absorbs heat from the surroundings. This type of reaction will typically result in a temperature decrease in the surrounding environment.
It’s important to note that enthalpy change aone is not always enough to determine the direction of the reaction. Other factors such as entropy and Gibbs free energy also need to be considered to fully understand the thermodynamics of a reaction.
Identifying Endothermic Reactions
To determine if a chemical reaction is endothermic, one must analyze the energy levels of the products and reactants involved in the reaction. If the products have a higher energy level than the reactants, it means that energy was absorbed during the reaction, which is a characteristic of an endothermic reaction.
Another way to identify an endothermic reaction is to observe if heat neds to be added to the reactants for the reaction to occur. If energy needs to be added to the system to initiate and maintain the reaction, it is most likely endothermic. In contrast, exothermic reactions release energy in the form of heat, light, or sound, and do not require additional energy to begin or continue the reaction.
A change in temperature during the reaction can also indicate whether it is endothermic or exothermic. If the temperature decreases, it is usually a sign of an endothermic reaction as the energy is absorbed from the surroundings, resulting in a cooling effect. Alternatively, if the temperature increases, it is most likely an exothermic reaction, as energy is released into the surroundings, leading to a warming effect.
Conclusion
In conclusion, an endothermic reaction is a chemical reaction that absorbs heat energy from its surroundings. This type of reaction requires an input of energy to create new chemical bonds and the energy is absorbed as a reactant. The surroundings feel colder as the heat is absorbed by the reaction. Endothermic reactions are important in many natural and industrial processes like photosynthesis, melting of ice, and cooking noodles. Understanding the principles of endothermic reactions is crucial in many fields of science, especally in chemistry and thermodynamics. It is essential to control the conditions of an endothermic reaction to optimize the desired outcome, such as in chemical synthesis or food processing. Overall, endothermic reactions play a critical role in our daily lives, and their study helps us to comprehend the fundamental principles of energy transfer and chemical reactions.
