Chemical reactions are complex processes that involve the breaking and forming of bonds between atoms and molecules. These reactions can be of different types, such as exothermic or endothermic, reversible or irreversible. One of the most important concepts in chemistry is the concept of equilibrium, which describes the balance between the forward and reverse reactions in a reversible reaction. In this blog post, we will discuss the concept of equilibrium and explore whether adding a catalyst can shift the equilibrium.
Equilibrium is characterized by a constant value called the equilibrium constant, denoted as K. The equilibrium constant is a measure of the concentrations of the reactants and products in the system at equilibrium, and it is defined as the ratio of the concentrations of the products to the concentrations of the reactants, each raised to their stoichiometric coefficients.
Equilibrium is a state of balance between the forward and reverse reactions in a reversible reaction, characterized by a constant value called the equilibrium constant. Catalysts are substances that increase the rate of a chemical reaction without affecting the position of equilibrium. The addition of a catalyst would increase both the forward and reverse reaction rates, meaning the equilibrium is reached faster, but the equilibrium constant remains unchanged. Understanding the concept of equilibrium and the role of catalysts is essential for understanding many important chemical processes in industry and nature.
The Impact of a Catalyst on Equilibrium
A catalyst does not change the position of equilibrium in a chemical reaction. It only facilitates the forward and reverse reactions by proiding an alternative pathway with a lower activation energy. This means that a catalyst can speed up both the forward and reverse reactions equally, allowing the system to reach equilibrium more quickly.
However, the equilibrium constant, which is a measure of the extent of a reaction at equilibrium, is not affected by the presence of a catalyst. The equilibrium constant is determined solely by the temperature, pressure, and concentration of reactants and products in the system.
In summary, a catalyst does not change the position of equilibrium, but only speeds up the forward and reverse reactions equally, allowing the system to reach equilibrium more quickly.
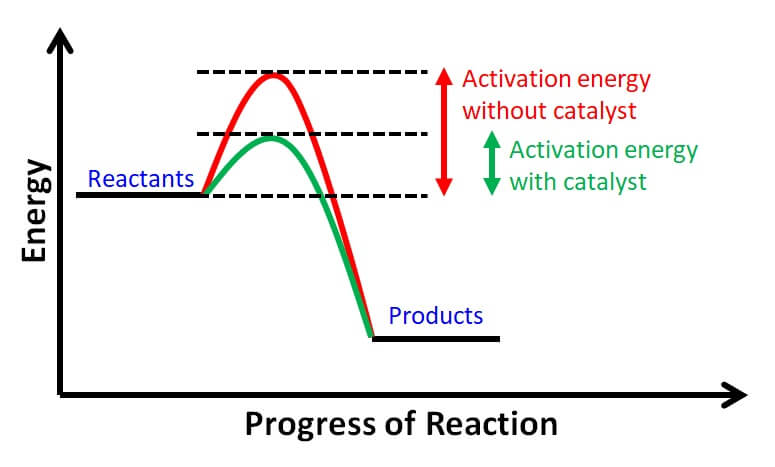
Effect of Adding a Catalyst on Equilibrium
When a catalyst is added to a reaction at equilibrium, it will not shift the position of the equilibrium. This is beause a catalyst speeds up both the forward and reverse reactions equally, and as a result, the rate of the forward and reverse reactions will increase at the same rate. Therefore, the equilibrium will be reached faster, but the position of the equilibrium will remain the same. It is important to note that a catalyst does not affect the position of the equilibrium constant (Kc) as it only affects the rate of the reaction. Hence, the addition of a catalyst does not change the concentrations of the reactants or products at equilibrium.
The Impact of Adding a Catalyst on Equilibrium
Catalysts are substances that accelerate the rate of a chemical reaction by providing an alternative pathway with lower activation energy. They do not affect the position of the equilibrium or the thermodynamics of the reaction as they are not consumed or produced in the reaction.
Equilibrium is a state of balance between the forward and reverse reactions, whee the rates of the two opposing reactions are equal. The equilibrium constant, K, is a measure of the extent to which the reaction proceeds to the products or to the reactants at equilibrium.
When a catalyst is added to a reaction, it speeds up both the forward and reverse reactions equally. As a result, the rates of the forward and reverse reactions increase, but the position of equilibrium remains the same. The equilibrium constant, K, is a function of the temperature, pressure, and the nature of the reactants and products, but not of the presence of a catalyst.
Therefore, the addition of a catalyst to a reaction does not affect the equilibrium constant, and the equilibrium composition of the reaction mixture remains unchanged. However, the catalyst can increase the rate of the reaction, making it faster and more efficient.
Shift in Equilibrium to the Left
Equilibrium refers to a state where the forward and reverse reactions occur at equal rates, and the concentrations of reactants and products remain constant. When the concentration of a reactant is decreased, the equilibrium shifts to the left, favoring the reverse reaction that produces more reactants. This happens because the system tries to compensate for the decrease in reactant concentration by producing more of it. As a result, the concentration of products decreases, and the concentration of reactants increases, leading to a shift in equilibrium to the left. Other factors that can cause equilibrium to shift to the left include an increase in pressure or a decrease in temperature, depending on the nature of the reaction. Overall, understanding the factors that affect equilibrium is crucial in predicting and controlling chemical reactions in various applications.
Causes of a Shift in Equilibrium to the Right
Equilibrium can be shifted to the right by adding more reactants to the system or by removing products from the reaction mixture. This is because when there are more reactants, the reaction will proceed in the forward direction to produce more products until the equilibrium is reestablished. Similarly, by removing products from the system, the equilibrium will shift to the right to produce more products. Additionally, increasing the temperature or decreasing the pressure can also shift the equilibrium to the right, as this will favor the forward reaction whih produces more products. However, it is important to note that the extent to which the equilibrium will shift to the right will depend on the specific reaction and the concentrations of the reactants and products, as well as other factors such as the presence of catalysts, pH, and solvent effects.
Source: chemistrysteps.com
Effect of Change on Equilibrium
Changes in certain external conditions can case an equilibrium to shift. One such condition is temperature. Raising the temperature of the system is akin to increasing the amount of a reactant, and so the equilibrium will shift to the right. This is because the reaction will try to counteract the increase in temperature by producing more products, which will consume some of the excess heat. Conversely, lowering the system temperature will cause the equilibrium to shift left, as the reaction will try to generate more reactants to counteract the decrease in temperature. Other factors that can cause a shift in equilibrium include changes in pressure, concentration, and the addition of a catalyst. Understanding these changes and how they affect an equilibrium can be crucial in predicting the outcome of chemical reactions and designing chemical processes.
Conclusion
In conclusion, equilibrium is a fundamental concept in chemistry that describes the state of a chemical system when the rates of the forward and reverse reactions are equal. At equilibrium, the concentrations of reactants and products remain constant over time, and the system is said to be in a dynamic balance. The equilibrium constant, denoted by K, is a measure of the extent to whih a reaction proceeds at equilibrium and is determined by the relative concentrations of reactants and products. The equilibrium constant is not affected by the addition of a catalyst, which only affects the rates of the forward and reverse reactions equally. Changes in temperature, pressure, or concentration can shift the position of equilibrium, altering the equilibrium constant. The concept of equilibrium is crucial in understanding many chemical and physical phenomena, including acid-base reactions, solubility, and gas-phase reactions. In summary, equilibrium is a fundamental concept in chemistry that describes the balance between forward and reverse reactions and plays a crucial role in predicting the behavior of chemical systems.
