The Dichromate Formula is an important component of chemical reaction processes and as such, it is vital to understand what this formula is and how it works.
In chemistry, a dichromate is a compound containing two chromium atoms in an oxidation state of +6. This type of compound is generally formed from the reaction of a chromium atom with oxygen or another oxidizing agent. The Dichromate Formula can be written as Cr2O7^2-. This formula describes the molecular structure of the compound and its properties.
The Dichromate Formula has several important uses in chemical reactions. For example, it can be used to oxidize certain organic compounds, such as alcohols, into aldehydes or ketones. It also has applications in the production of dyes, pigments, and other colorants. Additionally, it is used in water treatment processes to help remove heavy metals from wastewater.
The Dichromate Formula has several important safety considerations that should be taken into account before using it in any laboratory setting. First and foremost, it should never be handled without proper protective gear such as gloves, goggles, and a lab coat. Secondly, dichromates are extremely toxic compounds that can cause serious health problems if ingested or inhaled in large quantities. Therefore, all necessary precautionary measures must be taken when using them in any capacity.
Overall, the Dichromate Formula plays an important role in many types of reactions and processes within the field of chemistry. It is essential to understand its structure and its potential safety hazards so that one can use it safely and effectively in their experiments.
What Is The Formula For The Dichromate Ion?
The formula for the dichromate ion is Cr2O7-2. The ion is composed of one chromium atom and two oxygen atoms. Chromium is a transition metal that can form multiple ions with diferent charges. In the case of the dichromate ion, the charge is negative two. This means that the chromium atom has lost two electrons. The oxygen atoms each have a negative charge of one.
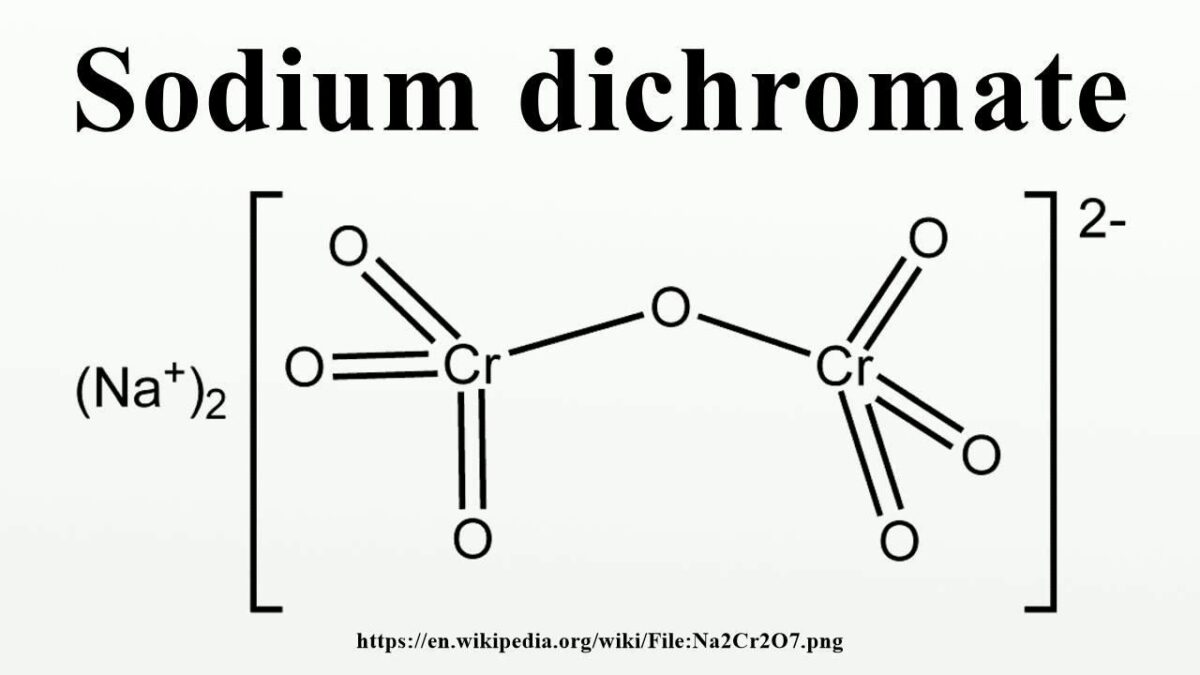
Why Is It Called Dichromate?
A dichromate is an oxyanion of chromium consisting of two chromium atoms and seven oxygen atoms, Cr2O7 2-. The name “dichromate” coes from the two different colors that this ion can form: orange when it is oxidized and green when it is reduced.
What Is Cr2O7 In Chemistry?
The chromate ion, CrO4 2-, is a polyatomic ion with the empirical formula Cr2O7. It consists of one chromium atom and seven oxygen atoms. The chromate ion is a strong oxidizing agent.
What Colour Is Dichromate?
Dichromate is a crystalline ionic solid that has a very bright, red-orange color. This compound is made up of chromium (III) ions and oxygen (II) ions, and it gets its color from the presence of the chromium ion. Chromium (III) is a transition metal that has a characteristic orange color, and when it is combined with oxygen, it produces a bright red-orange color.
What Is Dichromate Used For?
Potassium dichromate is used for a variety of purposes, the most common of whch are in glassware cleaning and etching. It can also be used in leather tanning, photographic processing, cement production, and wood staining.
What Means Dichromate?
Dichromate is a chromium salt that contains the anion Cr2O7 2-. It is usually orange to red in color.
How to Write the Chemical Formula for Chromate ion
What Color Is Cr2O7?
Chromium (III) oxide, Cr2O7, is a dark orange color. It is a strong oxidizing agent and is used in the production of chromate and dichromate salts. These salts are used in dyeing and chrome plating.
Is Cr2O7 A Base?
Yes, Cr2O7 is a base. It is a conjugate base of a hydrogen dichromate.
How Many Electrons Does Cr2O7 2 Have?
The chromium(VI) oxide ion has 18 electrons in its outer shell. It needs six more electrons to fill its octet, so it takes two oxygen atoms to share their eight electrons with the chromium ion. This makes the chromium(VI) oxide an anion, Cr2O7 2 -.
What Is K2cr2o4?
Potassium chromate is an inorganic compound with a chemical formula K2CrO4. It is also known as dipotassium monochromate or bipotassium chromate. It appears as a crystalline solid which is yellow in colour. It has no smell and has a disagreeable bitter taste.
Potassium chromate is most commonly used as a corrosion inhibitor in water treatment and as a mordant in dyeing textiles. It can also be used as a pigment for paints and inks.
What Is Perchloric Acid Used For?
Perchloric acid is a strong mineral acid that is used in various industries and laboratory settings. It is most often used to separate potassium from sodium, as the two elements have different boiling points and react differently with perchloric acid. In addition, perchloric acid is commonly used in tests to measure the amount of water in a substance, to determine the purity of metals, and to detect certan impurities. Perchloric acid can also be used to produce explosives and plating metals. However, it is a dangerous chemical that can explode if not handled properly, and it also produces toxic and corrosive fumes when heated.
Why Is Perchloric Acid The Strongest Acid?
Perchloric acid is the strongest acid because it has a higher electron affinity than sulfuric acid. This means that perchloric acid is more likely to donate an electron to aother molecule, making it more acidic. Additionally, perchloric acid has more double bonds than sulfuric acid, making it more stable.
What Is NH4 Called?
The NH4 ion is called ammonium. This ion has a positive charge and a molecular weight of 18g/mol. The nitrogen content of the ammonia, NH3-N, is the amount of nitrogen in the molecule. The nitrogen content of the ammonium ion, NH4-N, is the amount of nitrogen in the ammonium ion.
What Is The Formula Of Ammonium Chloride?
The formula of ammonium chloride is NH4Cl. It is an inorganic compound that is a white crystalline salt that is highly soluble in water.
