Diborane is a chemical compound composed of two boron atoms and six hydrogen atoms, with the molecular formula B2H6. It has a molar mass of 27.66 grams per mole, meaning that one mole of diborane weighs 27.66 grams. This is an important property to consider when measuring out and using the compound in chemical reactions or experiments.
Diborane can be produced in various ways, including the reaction between a metal hydride and boron, iodine and sodium borohydride in diglyme, or magnesium boride with hydrochloric acid. It is also knon as Boron hydride, Diboron hexahydride, Boroethane, and UN 1911.
Due to its unique structure, diborane has many uses in both industrial and laboratory settings. For instance, it is used as a fuel for rocket propulsion systems due to its high energy content and ability to burn at low temperatures. In addition, it can be used as a catalyst for organic synthesis reactions such as hydroboration-oxidation reactions used to make alcohols from alkenes.
In summary, diborane is an important compound with many applications due to its molar mass of 27.66 grams per mole and wide range of industrial uses. Its IUPAC Standard InChIKey is GCXMMZWJUYHRTG-UHFFFAOYSA-N and its CAS Registry Number is 19287-45-7.
Number of Moles in B2H6
The molar mass of diborane (B2H6) is 27.66 grams per mole, so if we have a certain mass of B2H6, we can use the following equation to calculate the number of moles: moles = mass / molar mass. For example, if we have 54.32 grams of B2H6, then the number of moles is 54.32 / 27.66 = 1.96 moles.
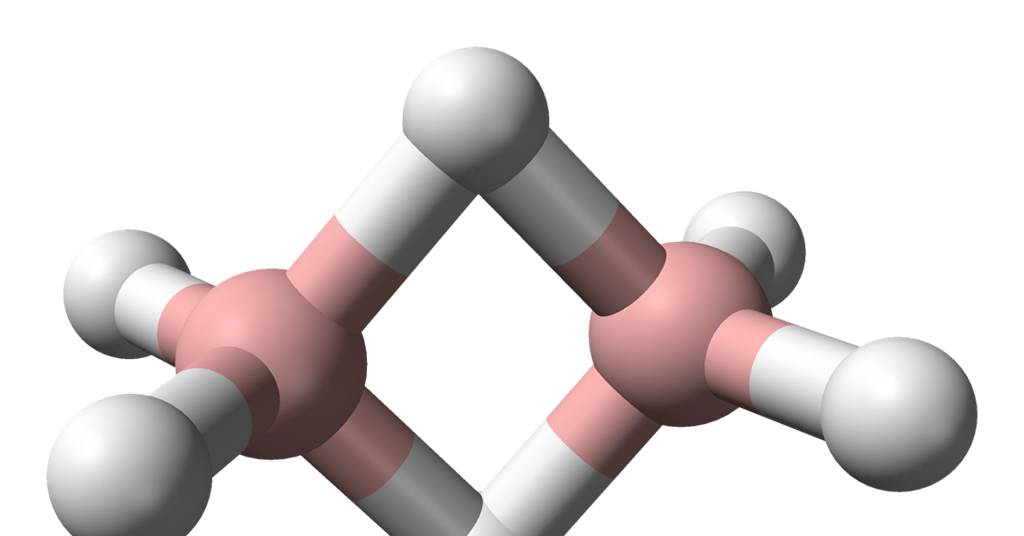
Number of Atoms in B2H6
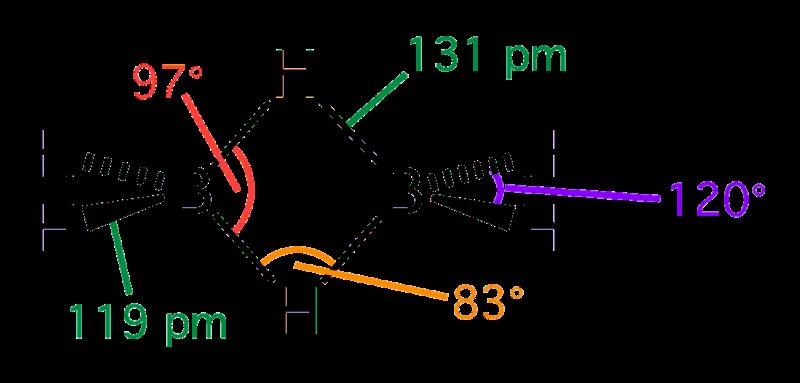
In the molecular formula of diborane, B2H6, there are a total of 8 atoms. This structure consists of two boron atoms (B) and six hydrogen atoms (H). Out of the six hydrogen atoms, four are known as terminal hydrogens and two are bridged hydrogens. All these atoms are arranged in a plane to form a stable molecule.
Formation of B2H6
Diborane (B2H6) is typically formed through the reaction of a metal hydride and boron. In this process, the metal hydride acts as a reducing agent as it donates two hydrogen atoms to the boron, forming B2H6. This reaction is exothermic, releasing energy in the form of heat. Additionally, diborane can be formed through the reaction of iodine with sodium borohydride in diglyme, although this method only produces small quantities. Lastly, heating magnesium boride with HCl yields a mixture of volatile boranes containing diborane.
IUPAC Name for B2H6
The IUPAC (International Union of Pure and Applied Chemistry) name for B2H6 is diborane(6), also known as boron hydride, diboron hexahydride, boroethane, or UN 1911. It is a colourless and odourless gas at room temperature and is composed of two boron atoms bonded with six hydrogen atoms. Its CAS registry number is 19287-45-7.
Molar Mass of B2O3
The molar mass of B2O3 is 69.617 g/mol. It is also known as boric oxide, boron trioxide, diboron trioxide, or boron sesquioxide and has the molecular formula B2O3. Its IUPAC name is oxo(oxoboranyloxy)borane.
Source: en.wikipedia.org
Does B2H6 Exist?
Yes, B2H6 does exist. It is the chemical compound known as diborane, which is a colorless and pyrophoric gas with a repulsively sweet odor. Diborane is highly toxic and flammable and should be handled with extreme caution. It was first discovered in 1899 by Walther Nernst, who synthesized it by passing hydrogen over a heated mixture of boron and magnesium. Since then, it has been used in various industrial processes such as semiconductor fabrication and rocket propellant production.
Bond Type of B2H6
B2H6 is a covalent bond. This type of bond is formed when two atoms share electrons in order to form a stable bond. In B2H6, two boron atoms use four of their valence electrons to form two covalent bonds with the six hydrogen atoms, forming a molecular structure of two Boron–Hydrogen bridges. The bonds between the Boron and Hydrogen atoms are strong, non-polar covalent bonds with no partial charges, making them relatively stable molecules.
Bond of B2H6
B2H6 is a molecule composed of two boron atoms and six hydrogen atoms. It has both covalent bonds, which are formed when two atoms share electrons, and hydrogen bridged bonds, which are formed when one hydrogen atom is shared between two other atoms. The boron atoms in B2H6 form covalent bonds with the six hydrogen atoms to complete their octets of valence electrons. The hydrogen bridged bonds form in between the boron atoms, where one oxygen atom is shared between them. In total, B2H6 contains four covalent bonds and two hydrogen bridged bonds.
Similarity of B2H6 and C2H6
No, B2H6 is not similar to C2H6. While both molecules contain two atoms of the same element and six hydrogen atoms, their molecular structures are quite different. C2H6 has 14 valence electrons, allowing it to form seven 2c−2e bonds, resulting in a linear structure. On the other hand, B2H6 only has 12 valence electrons, which limits it to forming a maximum of six 2c−2e bonds. As such, B2H6 requires the use of multicenter bonding to rationalize its structure.
What Makes B2H6 a Banana Bond?
B2H6 is a banana bond because it consists of two B–H–B bridging bonds, each of which is made up of three atoms (boron and hydrogen) sharing two electrons. This type of bonding, which is not linear but curved, is also known as a three-center-two-electron bond. The curved shape reminds some people of a banana, hence the name.
The Purpose of BH3 Existing as B2H6
Boron is a Group 13 element which has an incomplete octet due to its small size and hence, it cannot form BH3. To overcome this electron deficiency, BH3 dimerises to form B2H6, otherwise known as diborane. This is because the two Boron atoms in the molecule share their electrons and hence, each Boron atom has a complete octet. As a result of this electron sharing, each Boron atom also takes up two additional Hydrogen atoms. Thus, for stability reasons, BH3 exists as B2H6.
Are BH3 and B2H6 the Same?
No, BH3 and B2H6 are not the same. BH3 is a borane, which is a compound containing only boron and hydrogen atoms. It has the formula BH3 and is often referred to as diborane or dihydridoborane. On the other hand, B2H6 is an organoboron compound, meaning that in addition to boron and hydrogen atoms it also contains carbon atoms. It has the formula (B2H6) or 9-BBN, and is sometimes referred to as dialkylborane or dialkyldihydridoborane. Despite their different chemical structures, both compounds behave in similar ways and can be used in hydroboration reactions.
The Truth About B2H6
B2H6, also known as diborane, is a colourless, odourless gas composed of two boron atoms and six hydrogen atoms. It is an example of a Lewis acid-base reaction in which the boron acts as the Lewis acid and the hydrogen acts as the Lewis base. The molecule has a trigonal planar structure with all B-H-B angles at 120°. All terminal bonds have less p-character compared to bridging bonds due to the symmetric nature of the molecule. Due to its highly reactive nature, it is often used as an intermediate in organic synthesis reactions.

The Point Group of B2H6
The B2H6 point group is a molecule belonging to the D2h Point group that has three C2 rotation axes and three σ planes of symmetry. It contains two boron atoms (B) and six hydrogen atoms (H) and is a symmetric, non-polar molecule. The bond angles between each of the atoms are all equal to 90 degrees, thus giving it a linear shape. The molecule is also optically inactive, meaning that it does not rotate light plane-polarized light. This makes B2H6 an important compound for many industrial applications, such as welding or rocket fuel.
Conclusion
In conclusion, diborane is a chemical compound composed of two boron atoms and six hydrogen atoms with a molar mass of 27.66 grams per mole. Diborane can be produced through the action of metal hydrides with boron, the reaction of iodine with sodium borohydride in diglyme, or by heating magnesium boride with HCl. It has an IUPAC Standard InChIKey of GCXMMZWJUYHRTG-UHFFFAOYSA-N and a CAS Registry Number of 19287-45-7.
