When it comes to magnetism, most people think of materials that are attracted to magnets, such as iron or steel. But there is a class of materials that actually repel magnets – these are called diamagnetic elements.
Diamagnetic elements are materials that produce an induced magnetic field in the opposite direction of an externally applied magnetic field. This means that when a magnet is brought near them, they will be repelled instead of attracted.
The most strongly diamagnetic material is bismuth, with a susceptibility value of −1.66×10− 4. Pyrolytic carbon may have a susceptibility of χv = −4.00×10− 4 in one plane and superconductors are the perfect diamagnetic materials as they expel all the external magnetic field. Other examples of diamagnetic materials include wood, copper, gold, mercury, silver, lead and neon.
Diamagnetism occurs when electrons in atoms or molecules arrange themselves so that their individual fields cancel each other out – this creates an opposing field which repels the external magnetism. Interestingly enough, all matter has some degree of diamagnetism – however it is usually very weak compared to other forms of magnetism so it only becomes noticeable when placed in strong magnetic fields such as those produced by powerful magnets or superconductors.
In addition to being used for scientific studies and experiments relted to magnetism, some uses for diamagnetic elements include medical imaging (such as MRI) and levitation experiments. Diamagnets can also be used for shielding against external magnetic fields in sensitive environments such as hospitals and labs where strong external magnetic fields can interfere with certain processes or equipment.
Diamagnetic elements are fascinating materials with many intriguing properties!
Identifying Diamagnetic Elements
In order to determine if an element is diamagnetic, you must first look at its electron configuration. If all of the electrons in the element are paired, then it is diamagnetic. This means that when placed in a magnetic field, it will experience a force that opposes the field and be repelled by it. If any of the electrons are unpaired, then it is paramagnetic and will be attracted to the magnetic field.
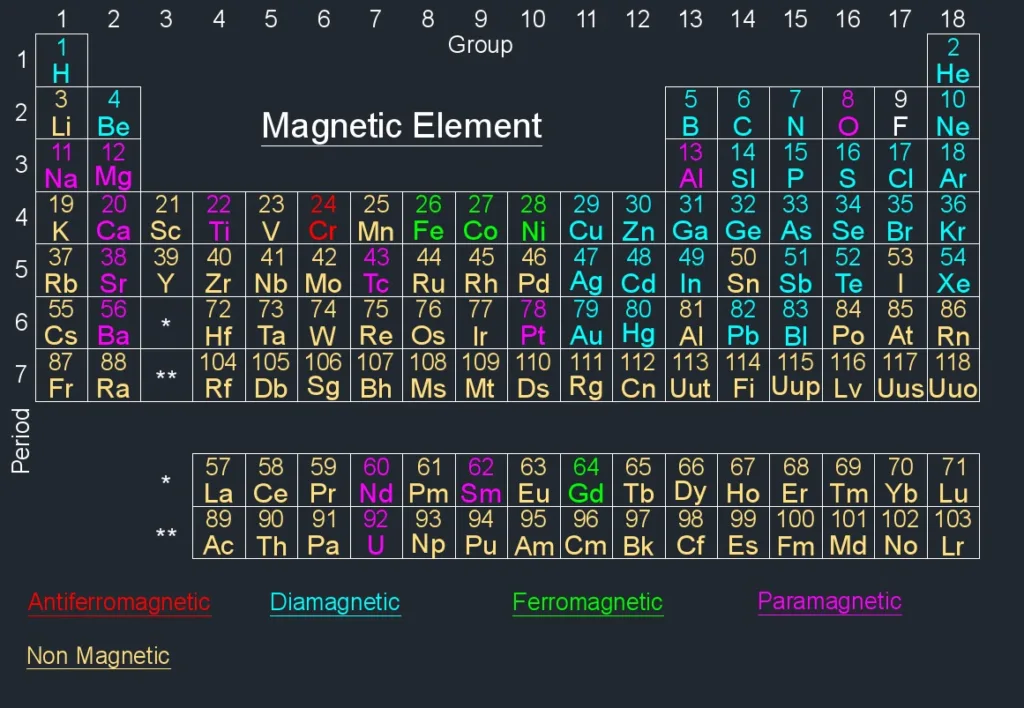
Examples of Diamagnetic Materials
Diamagnetic materials are substances that have an induced magnetic field in the opposite direction of an external magnetic field. This is due to the fact that all electrons within a diamagnetic material are paired together and therefore not available to be aligned with the external field. Examples of diamagnetic materials include wood, copper, gold, bismuth, mercury, silver, lead, neon, water and any superconductor. Superconductors are perfect diamagnets as they can completely repel any external magnetic field.
The Most Diamagnetic Element
The most diamagnetic element is bismuth, with a susceptibility of χv = −1.66×10− 4. Diamagnetism is a type of magnetism that occurs in materials that are repelled by an externally-applied magnetic field. It is the weakest form of magnetism and is present in all materials, although its effect can be very small in some cases. Bismuth stands out among other elements due to its particularly strong diamagnetic response, with a susceptibility value much higher than other materials or elements. Pyrolytic carbon may also have a very strong diamagnetic response in one plane, but bismuth still remains the most diamagnetic element overall.
Properties of Diamagnetic Metals
Diamagnetic metals are materials that display a weak repulsion to an externally applied magnetic field. This is in contrast to ferromagnetic materials, which are strongly attracted to magnetic fields. Examples of diamagnetic metals include aluminum, bismuth, gold, lead, and some alloys of nickel and cobalt. These materials don’t retain magnetic properties when the external field is removed. Other substances such as water and sugar are also considered to be diamagnetic even though they aren’t metals.
Understanding the Properties of Diamagnetic Elements
Diamagnetism is a type of magnetism that is found in all materials, and is characterized by the repulsion of a magnetic field. This repulsion occurs when the electrons in an atom’s orbitals are paired up and all have the same spin. This means that there are no unpaired electrons, which results in the material not having any net magnetic moment. As a result, when a material is placed in a magnetic field, it will be repelled instead of attracted. Diamagnetic materials are completely non-magnetic, meaning they cannot be magnetized or used to create permanent magnets.
Is Copper Diamagnetic?
Yes, copper (Cu) is diamagnetic. This means that it is affected by a magnetic field in such a way that it creates an opposing field, which causes the material to be repelled by the magnet. This occurs because the electrons in the atoms of copper are arranged in such a way that they have no net magnetic moment when placed in an external magnetic field. Copper’s diamagnetism makes it useful for applications where strong magnetic fields need to be neutralized or shielded, such as MRI machines and certain nuclear reactors.
Is Zinc Diamagnetic?
Yes, Zinc atoms are diamagnetic because they contain no unpaired electrons. All of the electrons in a Zinc atom occupy orbitals with opposite spins, which means that the total magnetic moment of the atom is zero. This type of behavior is characteristic of diamagnetic materials.
Is Gold Diamagnetic?
Yes, gold is diamagnetic, meaning it exhibits only a weak response to an external magnetic field. This means that in the presence of a magnetic field, gold will not be attracted or repelled by it. Instead, the electrons within the gold atoms will align themselves so as to create an opposing magnetic field. While this effect is very small and can only be detected with specialized equipment, it does mean that bulk gold is diamagnetic.
Is Lithium Diamagnetic?
Yes, Lithium is diamagnetic. This means that it is not attracted to a magnetic field. This is because there are no unpaired electrons in the atom’s electron shells, which are necessary for a material to be attracted to a magnetic field. Instead, all of the electrons are paired and spin in opposite directions, creating a net cancellation effect that prevents the atom from being attracted to a magnetic field.
Source: thoughtco.com
The Strength of Diamagnetism
The strongest diamagnetic material is Bismuth. It has a relatively high susceptibility to magnetic fields, which makes it ideal for use in guns and other applications where a strong magnetic field is required. Bismuth can be melted down and formed into any shape, allowing it to efficiently capture any diamagnetic properties. Another strongly diamagnetic material is Pyrolytic Graphite, which has the unique property of being able to be stably floated on a magnetic field, with a susceptibility as high as 162.
Which Substance Is Diamagnetic?
Diamagnetism is the property of a material or substance to be repelled by a magnetic field. Diamagnetic materials, such as copper and aluminum, have no unpaired electrons, so their electron clouds are symmetrically arranged. This means that when exposed to a magnetic field, the electron clouds repel the external field and the material is not attracted to it. The most common example of a diamagnetic material is hydrogen (H2). Hydrogen has two electrons that are paired in its molecular orbitals, so it is a diamagnetic molecule.
Is Calcium Diamagnetic?
No, calcium is not diamagnetic. While most materials, such as water, copper, nitrogen, and barium sulfate are diamagnetic, calcium atoms have paired outer-shell electrons and are thus paramagnetic. This means that when placed in a magnetic field, they will experience a weak force that is either attracted or repelled from the magnet depending on the orientation of the magnetic field.
Is Graphite Diamagnetic?
Yes, graphite is diamagnetic. It is an extremely strong diamagnet, wich means that it is repelled by a magnetic field rather than attracted to it. This effect is due to its structure; graphite consists of many layers of strongly bonded carbon atoms arranged in a hexagonal lattice, each layer of which is a flat sheet of graphene. As a result, the electrons in the lattice can move freely in the plane of the graphene sheets but not perpendicular to them, resulting in a very high anisotropy of magnetic susceptibility. Consequently, when exposed to an external magnetic field, the electrons will rearrange their orbitals in such a way that they form a pattern that minimizes the energy associated with this external field – this anti-alignment produces the repulsive diamagnetic effect.

Conclusion
In conclusion, diamagnetic elements are materials that display a weak magnetic repulsion when exposed to an external magnetic field. These elements are characterized by having all of their electrons paired and therefore no free electrons available for magnetization. Examples of diamagnetic elements include wood, copper, gold, bismuth, mercury, silver, lead and neon. Bismuth is the most strongly diamagnetic material with a susceptibility of -1.66 x 10-4. Diamagnetism can be used to repel magnets and create a counterforce against external magnetic fields.
