Welcome to my blog post aout delta G units! Delta G (or “free energy change”) is a measure of the amount of energy available to do work. It is an important concept in thermodynamics, and is used to understand how energy flows through different systems.
In order to accurately measure Delta G, we need to use a unit which is standardised across different types of measurements. The most commonly used unit for Delta G is the kilojoule per mole (kJ/mol). This expresses the amount of energy released or absorbed during a chemical reaction in terms of moles – it provides us with a single, consistent measurement so that we can make direct comparisons between reactions.
Another important concept related to Delta G is equilibrium constant (Keq). This represents the ratio between the concentrations of products and reactants at equilibrium, and it is related to Delta G by the equation ΔG°=−RTlnK. If ΔG° 1, and products are favored over reactants at equilibrium. If ΔG° > 0, then K < 1, and reactants are favored over products at equilibrium.
It’s also important to note that Delta G is not the same as Delta H or Delta S – these are related concepts but are not interchangeable! Delta H (or enthalpy change) measures the amount of heat released or absorbed in a reaction, while Delta S (or entropy change) measures the amount of disorder created or destroyed in a reaction.
I hope this blog post has been informative and helped you gain an understanding of Delta G units! Thanks for reading!
Understanding Delta G Value
Delta G (or ‘Gibbs Free Energy’) is a measure of the energy available to do work, and is an important factor in many chemical reactions. It is defined as the difference between the enthalpy (H) and entropy (S) of a system, multiplied by the temperature (T). In oter words, Delta G = H – T*S. When Delta G is negative, it indicates that a reaction is thermodynamically favorable; when it is positive, the reaction is not favored. The magnitude of Delta G can be used to calculate the equilibrium constant (Keq) for a reaction. In general, Delta G can be thought of as a measure of how much energy must be input into a system for it to reach equilibrium.
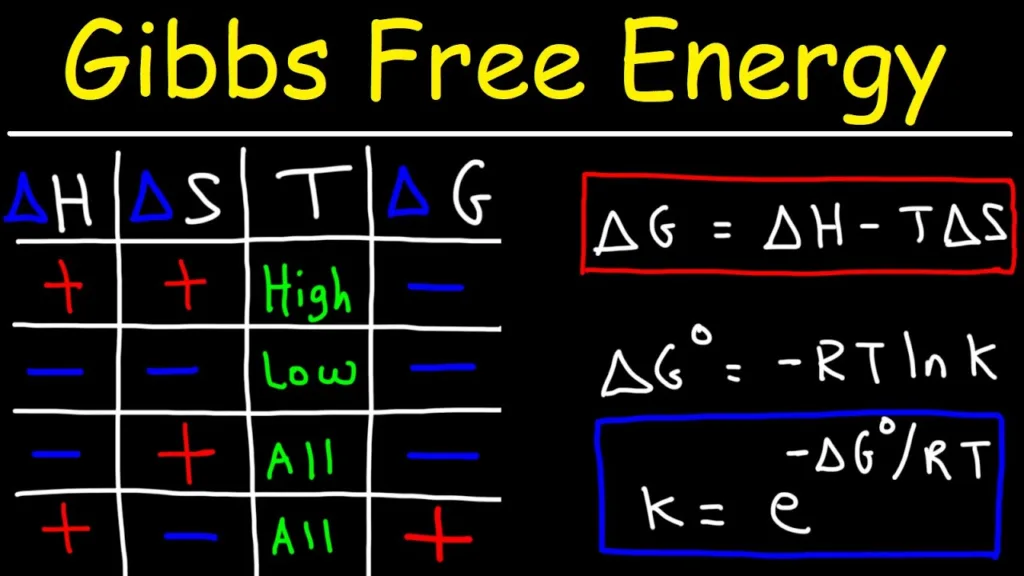
What is the Delta G Formula?
The Delta G formula, also knwn as the Gibbs Free Energy equation, is a thermodynamic equation that expresses the change in free energy of a system. It is expressed as ΔG = ΔH – TΔS, where ΔG is the change in free energy of the system, ΔH is the change in enthalpy (heat content) of the system, and TΔS is the product of temperature and entropy change in the system. This equation can be used to calculate how much energy will be released or absorbed during a reaction or process at a given temperature. The sign of ΔG dictates whether a reaction will occur spontaneously or not; if ΔG is negative then the reaction is spontaneous, while if ΔG is positive then the reaction is non-spontaneous.
Reading Delta G on a Graph
Reading delta G on a graph is a way of determining the spontaneity of a reaction. Delta G (ΔG) is the change in free energy, and it is denoted by the Greek letter Delta (Δ). A negative ΔG indicates that the reaction is exergonic and spontaneous, as it will favor a lower energy state. On a graph, ΔG is represented on the y-axis. The x-axis typically represents different points in time or concentrations of reactants and products. The slope of the line connecting thee points gives an indication of how much free energy has been released or absorbed during the reaction. A negative slope indicates that energy has been released, while a positive slope indicates that energy has been absorbed. A steep line means that a large amount of energy has been released or absorbed quickly, while a shallow line means that small amounts have been released or absorbed over time.
When Is Delta G Less Than Zero?
Answer: When the value of the Standard Gibbs Free Energy Change (ΔGo) is less than zero, it means that the reaction has a negative change in free energy (ΔG). This indicates that the reaction will spontaneously proceed and can be considered as thermodynamically favorable. In other words, a reaction with a negative ΔG will occur spontaneously and release energy.
What Units Are Used for Delta G Formula?
The ΔG formula is typically expressed in kJ/mol. This is because the units of ΔS (entropy) are typically expressed in J/K⋅mol, and kJ/mol is a more convenient unit for energy than J/mol. Therefore, it is necessary to convert ΔS from J/K⋅mol to kJ/K⋅mol before calculating ΔG.
The Relationship Between Delta G and Entropy or Enthalpy
Delta G is neither entropy nor enthalpy, but rather a combination of both. Delta G is the change in Gibbs Free Energy, which is calculated by subtracting the product of temperature and entropy from the change in enthalpy. This equation, Delta G = H – TS, shows that Delta G is dependent on both entropy and enthalpy, although it is not either one itself.
Understanding Delta G in Gibbs Free Energy
Delta G (ΔG) is the change in Gibbs Free Energy, a thermodynamic quantity that combines enthalpy and entropy into a single value. It is calculated as the sum of the enthalpy plus the product of temperature and entropy of the system. In other words, ΔG is a measure of how much energy is available to do work within a system. A negative ΔG indicats that the system can spontaneously release energy, while a positive ΔG indicates that external energy must be added to the system in order for it to release energy. In either case, the amount of energy released or required (ΔG) is a measure of how far away from equilibrium the system is.
Conclusion
In conclusion, ΔG units are a measure of the change in free energy between reactants and products in a reaction. They are related to the equilibrium constant K by the equation ΔG°=−RTlnK. If ΔG° is less than zero, then the products are favored over reactants at equilibrium. Conversely, if ΔG° is greater than zero, then the reactants are favored over products at equilibrium. Thus, ΔG can be used to predict whether a reaction will proceed in a particular direction or not.
